Article Text
Abstract
Objective To evaluate changes in the outcomes of infants born at <25 weeks’ gestation in the past decade.
Design Retrospective observational study.
Settings A multicentre database of the Neonatal Research Network, Japan.
Patients A total of 3318 infants born at 22–24 weeks’ gestation between periods 1 (2003–2007) and 2 (2008–2012) from 52 tertiary centres.
Main outcome measures We compared death and neurodevelopmental impairments (NDIs) at 3 years of age, including cerebral palsy (CP), visual impairments (VIs), hearing impairments (HIs) and the developmental quotient (DQ) of the Kyoto Scale of Psychological Development test <70, between two periods using multivariate logistic regression analyses adjusted for the centre, gender, multiple gestation, maternal age, caesarean delivery, antenatal steroid use, pregnancy-related hypertension, clinical chorioamnionitis, congenital anomalies and birth weight.
Results A total of 496/1479 infants (34%) in period 1 and 467/1839 (25%) in period 2 died by 3 years of age (adjusted OR 0.70, 95% CIs 0.59 to 0.83). Follow-up data were collected from 631 infants (64% of survivors) in period 1 and 832 (61% of survivors) in period 2. The proportions of CP with Gross Motor Function Classification System ≥2, VI and HI in the infants evaluated were lower, while that of DQ <70 was higher in period 2 than in period 1. Using multiple imputations to account for missing data, death or NDI decreased from 54% in period 1 to 47% in period 2 (0.83, 0.71 to 0.97). Significant decreases were observed in death or CP (0.65, 0.55 to 0.76), death or VI (0.59, 0.50 to 0.69) and death or HI (0.69, 0.58 to 0.81), but not in death or DQ <70 (0.91, 0.78 to 1.06).
Conclusion Along with improved survival, CP, VI and HI, but not cognitive impairments decreased in infants born at <25 weeks’ gestation between the two periods examined in the last decade. Further strategies are needed to reduce cognitive impairments in these infants.
- neurodevelopment
- neonatology
- outcomes research
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Statistics from Altmetric.com
What is already known on this topic?
With active treatment, the survival of infants born at <25 weeks’ gestation has increased in the past decade.
The incidence of death or neurodevelopmental impairments in a Japanese cohort in 2003–2005 was 80% in infants at 22 weeks.
What this study adds?
Death or neurodevelopmental impairments in infants born at <25 weeks’ gestation decreased from 54% in period 1 (2003–2007) to 47% in period 2 (2008–2012).
Significant decreases were observed in death or cerebral palsy, death or visual impairments, and death or hearing impairments, but not in death or cognitive impairments.
Introduction
The mortality rates of extremely preterm infants born at a gestational age (GA) of 22 and 23 weeks are high, and those who survive often have neurological and developmental impairments.1–4 We previously reported the outcomes of 1057 infants born at 22–25 weeks’ gestation between 2003 and 2005 in the Neonatal Research Network of Japan (NRNJ).5 The incidence of death or neurodevelopmental impairments (NDIs) at a chronological age of 36–42 months was 80% of those born at 22 weeks and 64% at 23 weeks. In the subsequent 10 years, survival among these infants has increased in the Japanese cohort and those of other countries.6–9 Improvements in survival raise concerns among physicians, families and the public regarding increases in NDI in survivors. Precise information on survival and neurodevelopmental outcomes is needed in order to counsel parents and make decisions to provide active care. Changes in outcomes may correlate not only with prenatal characteristics, but also neonatal care, interventions and morbidities in neonatal intensive care units (NICUs). The aim of the present study was to evaluate changes in the 3-year outcomes of infants born at <25 weeks’ gestation in tertiary centres in Japan in the past 10 years. The survival and NDI in infants born and cared for at 52 centres were retrospectively compared between the first and second periods.
Methods
Study design, subjects and definition
We performed a multicentre, retrospective analysis using the NRNJ database.6 10 Each participating centre registered all live-born very low birthweight infants who were admitted to the NICU from 2003 to the database. Live-born infants who were cared for at delivery rooms but not admitted to the NICU started to be registered from 2006. The number of participating centres increased from 38 to 103 centres during the study period. In order to compare the outcomes of infants from the same tertiary centres between the two periods, 2003–2007 (period 1) and 2008–2012 (period 2), any centre that did not recruit 10 infants in both the first and second periods were excluded from this study. Infants transferred to centres after birth were also excluded in order to reduce selection bias if only infants in good condition or those who needed specific treatments, such as patent ductus arteriosus (PDA) ligation, were transferred after birth.11 Therefore, study subjects were infants born at 22 weeks 0 days to 24 weeks 6 days between 1 January 2003 and 31 December 2012 at 52 tertiary centres in Japan and admitted to the NICU of the same centres.
GA was obtained from obstetric histories with confirmation or corrections using ultrasonography at health check-ups for pregnant women during the first trimester. Demographic, prenatal and neonatal data were collected from each centre using previously described definitions.5 6 10 Active treatment for infants was defined if they received any of the following interventions: tracheal intubation, ventilator support including continuous positive airway pressure, surfactant therapy or parental nutrition.12 Data on epinephrine use or chest compressions were not available in the NRNJ database.
Outcomes
Death or NDI at 3 years of age was the primary outcome. Neonatal death was defined as infants who were born alive but died in the delivery room or NICU before discharge from centres registered to the database. Death after discharge was all deaths after discharge from centres to a chronological age of 42 months including death at facilities and at home.
A comprehensive neurodevelopmental assessment was performed on surviving infants at a chronological age of 36–42 months by a trained paediatrician at each centre, who was not necessarily blinded to perinatal details, as previously reported.5 Cerebral palsy (CP) was defined as a non-progressive, non-transient central nervous system disorder characterised by abnormal muscle tone in at least one extremity and the abnormal control of movement and posture.13 Children with any type of CP who were defined as Gross Motor Function Classification System (GMFCS) level 1 were excluded from the CP group because they were functionally not impaired or minimally impaired.14 Visual impairments (VIs) were defined as blindness with no functional vision in at least one eye or bilateral amblyopia. Bilateral amblyopia was diagnosed when children responded to light or hand movement, but not to other movements or objects regardless of the use of glasses. Hearing impairments (HIs) were defined when amplification was required. Cognitive functions were assessed using the Kyoto Scale of Psychological Development (KSPD) test.15 The latest version of the KSPD was standardised in 2001 for Japanese children, and the mean and 1 SD of the developmental quotient (DQ) were 100.6 and 13.4, respectively. A DQ score of KSPD <70, which represents a 70% achievement of standardised performance for the chronological age, was interpreted as significantly delayed according to the protocol by the Japan Neonatal Follow-up Study Group.16 A DQ score of KSPD <70 is equivalent to a Bayley III cognitive score <85.17 This test was administered by certified psychologists blinded to perinatal details at each centre; however, they were aware that participants were extremely preterm infants.
NDIs were defined as any of the following: CP with GMFCS ≥2, VI, HI or cognitive impairments with DQ of KSPD <70.
Statistical analysis
The characteristics of study subjects and prenatal or neonatal morbidities were described as the mean and SD for continuous variables and a number and proportion for binary and categorical variables. Unadjusted comparisons between the two periods were performed using the χ2 or Fisher’s exact test for categorical data and the t-test for continuous data.
A change in death or NDI at 3 years of age among the whole study population between periods 1 and 2 was the primary analysis. We then compared death or each disability: CP with GMFCS ≥2, VI, HI and cognitive impairments as DQ of KSPD <70. We also analysed subgroup subjects by gestational weeks. Multivariate logistic regression analyses revealed adjusted ORs (AORs) and 95% CIs for period 2 versus period 1 adjusted for the following demographic and prenatal characteristics and interventions: the centre, gender, multiple gestation, maternal age, caesarean delivery, antenatal steroid use, pregnancy-related hypertension, clinical chorioamnionitis (CAM), life-threatening congenital anomalies and birth weight. These factors were maternal or pregnancy-related factors that were not able to change after birth, and they were identified as variables associated with outcomes in previous follow-up studies.18–22 Since neonatal factors were considered to be intermediate variates that correlate with prenatal factors and changes in outcomes, they were not included in logistic regression models.
We used multiple imputations with the assumption that data were missing at random in order to avoid selection bias from missing data.23 24 The imputation was modelled by the potential confounders used in the above logistic regression models and all outcomes including all deaths, CP with GMFCS ≥2, VI, HI and DQ of KSPD <70. As a sensitivity analysis, we performed single imputations in one scenario in which missing data were imputed as having impairments or another scenario in which missing data were imputed as having no impairments.
Statistical analyses were performed using SPSS V.23 (IBM Japan) and SAS V.9.2 (SAS Institute).
Results
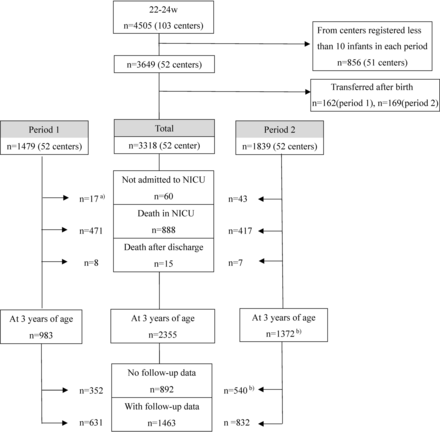
Study subjects comprised 3318 infants in 52 participating centres: 1479 in period 1 and 1839 in period 2 (figure 1). The number (%) of NICU admissions was 669/686 infants (97.5%) born in the last two years of period 1 (2006 and 2007) and 1796/1839 (97.7%) over 5 years in period 2. The annual numbers of live-born infants who were not admitted to the NICU, who died in the NICU and who received active treatment are shown in online supplemental table 1. Physicians at each centre decided to perform active treatment or withdraw care depending on the clinical status of infants; 1459/1479 infants (98.6%) in period 1 and 1804/1839 (98.0%) in period 2 received active treatment.
Supplementary file 1
Flow chart for study subjects in period 1 (2003–2007) and period 2 (2008–2012). a)This category started to be registered from 2006 in period 1. b)Two infants with missing data on death at neonatal intensive care unit (NICU) discharge in period 2 were estimated to have survived without follow-up data.
Table 1 shows comparisons of demographic and prenatal characteristics between the two periods. Antenatal steroid use markedly increased from 36.0% in period 1 to 53.3% in period 2. More infants were born via caesarean section and fewer infants were multiple births.
Comparison of demographic and prenatal characteristics of study subjects between periods
Common in-hospital morbidities and interventions are shown in table 2. Comparisons between the two periods using an unadjusted analysis showed that major neonatal morbidities all increased, whereas intraventricular haemorrhage (IVH) grades 3–4 significantly decreased. The proportion of cystic periventricular leukomalacia (PVL) slightly decreased. The proportion of infants receiving medical treatment or interventions in the NICU, such as treatments for PDA, late-onset circulatory collapse, chronic lung disease and retinopathy of prematurity, or receiving surfactant, antibiotics and parental nutrition as listed in table 2, significantly increased.
Comparison of in-hospital neonatal morbidities and interventions between periods
Follow-up data with assessments at 3 years of age were collected for 1463 infants: 631 (64.1% of survivors) in period 1 and 832 (60.7% of survivors) in period 2, including 39% full evaluations and 42% incomplete evaluations. Mean (SD) ages in months among infants at evaluations were 38.6 (2.6) months in period 1 and 38.0 (2.3) months in period 2. Differences in variables between infants with and without follow-up data are shown in online supplemental table 2. A significant difference was only observed in the proportion of clinical CAM.
Infant survival at 3 years increased from 66.5% (983/1479 infants) in period 1 to 74.6% (1372/1839 infants) in period 2. The proportions of impairments in infants with actual evaluations are shown in table 3. All proportions of CP with GMFCS ≥2, VI and HI in the evaluated infants were lower in period 2 than in period 1, whereas the proportion of DQ of KSPD <70 increased from 32.8% (126/384 measurements) in period 1 to 37.3% (250/670 measurement) in period 2. Among the 175 children not tested by KSPD, 36 (20.6%) had CP and 26 (14.9%) had VI. In contrast, among the 1054 children tested by KSPD, 67 (6.3%) had CP and 59 (5.6%) had VI. No significant changes were noted in NDI in infants with full evaluations or in those with incomplete evaluations between the two periods.
Comparison of survival and neurodevelopmental outcomes at 3 years of age for periods 1 and 2 before imputations
Survival and neurodevelopmental outcomes after multiple imputations are summarised in table 4. In period 1, 30.3% (n=298) of surviving children had NDI, while 28.8% (n=395) had NDI in period 2. These proportions were lower than those shown in table 3 in periods 1 and 2. In subgroup analyses by GA, NDI in survivors born at 22 weeks increased from 34.4% in period 1 to 46.4% in period 2, while that in survivors born at 23 and 24 weeks decreased between the two periods.
Survival and neurodevelopmental outcomes at 3 years of age for periods 1 and 2 after multiple imputations
Table 5 shows the AOR and 95% CI for death or each impairment in period 2 versus period 1 with adjustments by a multivariate logistic regression after multiple imputations. In all subjects, period 2 correlated with a decrease in death and death or NDI. Death or CP with GMFCS ≥2, death or VI, and death or HI also decreased. No significant change was observed for death or DQ of KSPD <70. In subgroup analyses by GA, all impairments significantly decreased in infants born at 23 weeks. In infants at 22 and 24 weeks, no significant changes were noted for death or NDI and death or DQ of KSPD <70. After single imputations (online supplemental table 3 and 4) in which missing data were imputed as having impairments, no significant changes were noted for death or NDI in all subjects or in any subgroups of GA. After single imputations in which missing data were imputed as having no impairments, death or NDI significantly decreased in all subjects and in infants born at 23 and 24 weeks.
Adjusted ORs for outcomes at 3 years of age in period 2 versus period 1 after multiple imputations
Discussion
This study showed improvements in the survival and neurodevelopmental outcomes of extremely preterm infants born at <25 weeks’ gestation between 2003 and 2012 from 52 tertiary centres in Japan who received active treatment throughout the study period. We observed changes in infant and maternal characteristics. Infants from multiple births decreased, while more mothers were complicated by pregnancy-related hypertension or clinical CAM, known risk factors for neurological impairments.20 21 25 More active prenatal interventions, such as antenatal steroid use and caesarean sections, which may improve conditions at birth, have been performed. Even after adjusting for these prenatal factors, death or NDI at 3 years of age significantly decreased in the second period. When study subjects were stratified by gestational weeks, improvements were observed in all categories in infants born at 23 weeks’ gestation. In infants at 22 weeks, no significant changes were found in death or NDI and death or DQ of KSPD <70. In surviving subjects, CP with GMFCS ≥2 and VI decreased between the two periods. However, the proportion of cognitive impairments slightly increased.
Among the types of disabilities observed in surviving infants, VI and CP decreased, and no improvement was found in cognitive impairments. Although the reason for this remains unclear, we suggest several causes: cognitive development is complex and affected by multiple factors, such as socioeconomic, environmental, inflammatory and nutritional factors.26–28 The extreme prematurity of the brain itself may be critical for later brain functions.29 In contrast, CP is directly related to brain damage as haemorrhagic or ischaemic brain injuries. We suspect that more strategies to prevent hypoxic, hyperoxic or ischaemic damage were performed in the second period.
Previous studies from England (EPICure) showed that the proportion of survival without disabilities was higher in infants born at 22–25 weeks’ gestation in 2006 than in those born at the same GA in 1995.4 Survival without disabilities in 2006 was 15% in infants born at 23 weeks admitted for neonatal care and 30% in those born at 24 weeks. The National Institute of Child Health and Human Development Neonatal Research Network recently reported increased rates of survival without NDI at corrected ages of 18–22 months for infants born at <25 weeks’ gestation between 2000 and 2011.30 The proportion of survival without NDI increased to 20% in the third period (2008–2011). Differences in the proportion of survival without disabilities were mostly explained by the survival proportions in this study. Our results are consistent with the finding showing that survival without disabilities has recently increased.
An investigation on the factors influencing NDI in these infants is important for further improvements, but is not the main purpose of the present study. Antenatal corticosteroid exposure is associated with a significant decrease in mortality.22 However, reductions in death or NDI and other impairments were significant after adjusting for antenatal steroid use. In our previous studies, cystic PVL, IVH grade 3–4, gastrointestinal perforation, sepsis, chronic lung disease and retinopathy of prematurity correlated with death or CP and death or developmental delays in very low birthweight infants.31 However, more infants were diagnosed with these unfavourable morbidities, except IVH and PVL, in period 2 than in period 1. By adjusting for prenatal factors, we speculate that improved outcomes are related to several interventions after birth such as parenteral nutrition in the NICU, which increased between the two periods, in addition to decreases in severe IVH and cystic PVL.
This study had several limitations. The first limitation was related to follow-up data, with actual assessments at 3 years of age being collected from 62% of survivors. Therefore, we used imputational techniques and estimated the outcomes of all of the study population. Although clinical details suggest that missing data were random, there may be biases from social, economic and other unknown factors. Although there was no significant difference in prenatal and neonatal variables between with and without follow-up data, based on comparisons of results between tables 3 and 4, the proportion of each impairment decreased after multiple imputations in both periods. The absolute percentage of impairments needs to be interpreted carefully. Therefore, we performed two single imputations for NDI. Significant changes in outcomes may differ if all missing data were from infants having impairments, as shown in online supplemental table 4.
The second limitation is that we selected centres that registered >10 infants born at <25 weeks’ gestation in each period because centres need to be matched between the two periods in order to compare changes in outcomes. The present results represent the outcomes of a selected population of extremely preterm infants cared for in tertiary centres in Japan. These subjects accounted for 34% in period 1 and 42% in period 2 among all infants born at 22–24 weeks’ gestation nationwide (online supplemental table 1).32 Further studies are needed in order to investigate between-centre variations in the outcomes of these extremely preterm infants.
The third limitation is that data on deaths in the delivery room were collected from 2006. The decision to withdraw/withhold intensive care in the delivery room depended on the physician at each participating hospital. Although the number of deaths in the delivery room was small, if deaths in the delivery room were accounted for from 2003, the mortality rate in period 1 may have been slightly higher and improvements in period 2 may have been greater. When excluding infants who died in the delivery room from the study subjects, the proportions of death in the NICU were 471/1462 (32.2%) in period 1 and 417/1794 (23.2%) in period 2.
Another limitation is a lack of registration of the socioeconomic status of the families of subjects or early interventions after discharge to support infants and families, which are known to affect developmental impairments and follow-up rates.24 33–35 Subsequent neurological outcomes as well as academic and social achievements need to be evaluated.
Conclusion
Infants born at <25 weeks’ gestation have received more prenatal and neonatal interventions in the last decade. Although bias due to the lack of follow-up data cannot be denied, along with improved survival, death or NDI, death or CP with GMFCS ≥2, death or VI, and death or HI, but not death or cognitive impairments with DQ <70 decreased following adjustments for prenatal factors. Further strategies are needed in order to reduce developmental or cognitive impairments in these infants.
Acknowledgments
Institutions enrolled in the study of the Neonatal Research Network, Japan, were as follows: Sapporo City General Hospital, Asahikawa Kosei General Hospital, Engaru-Kosei General Hospital, Kushiro Red Cross Hospital, Obihiro-Kosei General Hospital, Tenshi Hospital, NTT Higashinihon Sapporo Hospital, Nikko Memorial Hospital, Nayoro City General Hospital, Sapporo Medical University, Asahikawa Medical University, Aomori Prefectural Central Hospital, Iwate Medical University, Iwate Prefectural Ofunato Hospital, Iwate Prefectural Kuji Hospital, Iwate Prefectural Ninohe Hospital, Sendai Red Cross Hospital, Akita Red Cross Hospital, Tsuruoka Municipal Shonai Hospital, Yamagata University, Yamagata Prefectural Central Hospital, Fukushima Medical University, Takeda General Hospital, Fukushima National Hospital, Tsukuba University, Tsuchiura Kyodo Hospital, Ibaraki Children’s Hospital, Dokkyo Medical University, Jichi Medical University, Ashikaga Red Cross Hospital, Gunma Children’s Medical Center, Kiryu Kosei General Hospital, Fuji Heavy Industries Health Insurance Society Ota Memorial Hospital, Gunma University, Saitama Children’s Medical Center, Nishisaitama-chuo National Hospital, Saitama Medical University Saitama Medical Center, Kawaguchi Municipal Medical Center, Jichi Medical University Saitama Medical Center, Asahi General Hospital, Chiba Kaihin Municipal Hospital, Kameda Medical Center, Tokyo Women’s Medical University Yachiyo Medical Center, Juntendo University Urayasu Hospital, Tokyo Metropolitan Children’s Medical Center, Tokyo Women’s Medical University, Aiiku Hospital, Nihon University Itabashi Hospital, National Center for Global Health and Medicine, Tokyo Medical University, Teikyo University, Showa University, Japan Red Cross Medical Center, National Center for Child Health and Development, Tokyo Metropolitan Otsuka Hospital, Toho University, Tokyo Metropolitan Bokuto Hospital, Tokyo Jikei Medical University, Tokyo Medical and Dental University, Saint Luke’s International Hospital, Juntendo University, Sanikukai Hospital, Katsushika Red Cross Hospital, Yokohama Rosai Hospital, Yokohama City University Medical Center, St. Marianna University School of Medicine Hospital, Kanagawa Children’s Medical Center, Tokai University, Kitazato University, Odawara Municipal Hospital, Nippon Medical School Musashi Kosugi Hospital, Saiseikai Yokohamashi Tobu Hospital, National Hospital Organization Yokohama Medical Center, Yamanashi Prefectural Central Hospital, Nagano Children’s Hospital, Shinshu University, Iida Municipal Hospital, National Hospital Organization Shinshu Ueda Medical Center, Saku General Hospital, Niigata University, Niigata Prefectural Central Hospital, Niigata Municipal Hospital, Nagaoka Red Cross Hospital, Koseiren Takaoka Hospital, Toyama Prefectural Central Hospital, Toyama University, Ishikawa Medical Center for Maternal and Child Health, Kanazawa Medical University, Kanazawa Medical Center, Fukui Prefectural Hospital, Fukui University, Gifu Prefectural General Medical Center, National Hospital Organization Nagara Medical Center, Takayama Red Cross Hospital, Seirei Hamamatsu Hospital, Shizuoka Saiseikai Hospital, Shizuoka Children’s Hospital, Hamamatsu Medical University, Numazu Municipal Hospital, Yaizu City Hospital, Fujieda Municipal General Hospital, Nagoya Red Cross Daini Hospital, Nagoya University, Nagoya Red Cross Daiichi Hospital, Toyohashi Municipal Hospital, Nagoya City West Medical Center, Anjo kosei Hospital, Tosei General Hospital, Komaki Municipal Hospital, TOYOTA Memorial Hospital, Okazaki Municipal Hospital, Konan Kosei Hospital, National Mie Central Medical Center, Ise Red Cross Hospital, Yokkaichi Municipal Hospital, Otsu Red Cross Hospital, Shiga University of Medical Science Hospital, Nagahama Red Cross Hospital, Uji Tokushukai Hospital, The Japan Baptist Hospital, Kyoto University, Kyoto Red Cross Daiichi Hospital, National Maizuru Medical Center, Fukuchiyama City Hospital, Kyoto Prefectural University of Medicine Hospital, Kyoto City Hospital, Mitsubishi Kyoto Hospital, Yodogawa Christian Hospital, Osaka Medical Center and Research Institute for Maternal and Child Health, Osaka University, Takatsuki General Hospital, Kansai Medical University, Osaka City General Hospital, Osaka City Sumiyoshi Hospital, Aizenbashi Hospital, Toyonaka Municipal Hospital, National Cerebral and Cardiovascular Center, Kitano Hospital, Saiseikai Suita Hospital, Chifune Hospital, Bell Land General Hospital, Rinku General Medical Center, Osaka Red Cross Hospital, Yao Municipal Hospital, Osaka General Medical Center, Osaka City University, Hyogo Prefectural Kobe Children’s Hospital, Kobe University, Kakogawa West City Hospital, Saiseikai Hyogoken Hospital, Kobe City Medical Center General Hospital, Hyogo College of Medicine Hospital, Himeji Red Cross Hospital, Toyooka Public Hospital, Hyogo Prefectural Awaji Medical Center, Nara Medical University, Wakayama Medical University, Tottori Prefectural Central Hospital, Tottori University, Shimane Prefectural Central Hospital, Matsue Red Cross Hospital, Kurashiki Central Hospital, Tsuyama Central Hospital, Kawasaki Medical School Hospital, National Hospital Organization Okayama Medical Center, Okayama Red Cross Hospital, Hiroshima City Hiroshima Citizens Hospital, Hiroshima Prefectural Hospital, Hiroshima University, Tsuchiya General Hospital, National Hospital Organization Kure Medical Center, Yamaguchi University, Yamaguchi Grand Medical Center, Tokushima University, Tokushima Municipal Hospital, Kagawa University, National Hospital Organization Kagawa Children’s Hospital, Matsuyama Red Cross Hospital, Ehime Prefectural Central Hospital, Kochi Health Science Center, St. Mary’s Hospital, National Kyushu Medical Center, Kurume University, Kitakyushu Municipal Medical Center, University of Occupational and Environmental Health, Fukuoka University, Kyushu University, Iizuka Hospital, National Hospital Organization Kokura Medical Center, National Hospital Organization Saga Hospital, National Hospital Organization Nagasaki Medical Center, Kumamoto City Hospital, Kumamoto University, Oita Prefectural Hospital, Almeida Memorial Hospital, Nakatsu Municipal Hospital, Miyazaki University, National Hospital Organization Miyakonojo Medical Center, Kagoshima City Hospital, Imakiire General Hospital, Okinawa Prefectural Nanbu Medical Center & Children’s Medical Center, Okinawa Prefectural Chubu Hospital, Naha City Hospital, and Okinawa Red Cross Hospital.
References
Footnotes
Contributors YK conceptualised and designed the study and drafted the initial manuscript. NY performed the initial analyses and reviewed and revised the manuscript. HN set up the data and reviewed the manuscript. SK and MF initially conceptualised and designed the database, supervised the study and reviewed the manuscript. All authors agreed to be accountable for all aspects of the work.
Competing interests None declared.
Patient consent Parental/guardian consent obtained.
Ethics approval The study protocol was approved by the Ethics Review Committees of Tokyo Women’s Medical University and Jichi Medical University.
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement Data may be open for sharing if required.