Article Text
Abstract
Objective To describe haematocrit at birth in preterm infants who received ≥60 s of delayed cord clamping (DCC).
Design Retrospective observational study.
Setting A California public hospital with an American Academy of Pediatrics level 4 neonatal intensive care unit, with 3500–4000 deliveries annually.
Participants 467 preterm infants born at <35 weeks’ gestational age (GA) between January 2013 and December 2018.
Primary and secondary outcome measures Haematocrit reference ranges for 0–4 hours after birth and paired haematocrit differences between 0–4 and 4–24 hours.
Methods Haematocrits were obtained when clinically indicated and collected from arterial, venous and capillary sources. Haematocrits obtained after packed red blood cell transfusions were excluded. We summarised the first available haematocrit between 0 and 4 hours by GA strata. We used mixed-effects linear regression to describe the associations between haematocrit and predictor variables including GA, male sex and hours after an infant’s birth. We also compared paired haematocrits at 0–4 and 4–24 hours after birth.
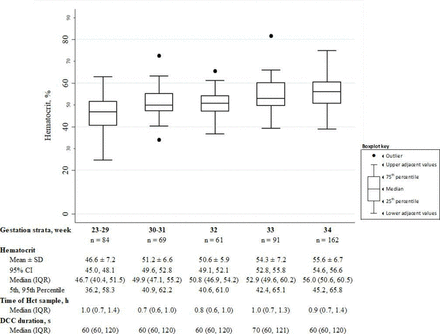
Results The median GA of the 467 included infants was 33.3 weeks, birth weight was 1910 g and DCC duration was 60 s. The mean (95% CI) first haematocrit at 0–4 hours was 46.6 (45.0% to 48.1%), 51.2 (49.6% to 52.8%), 50.6 (49.1% to 52.1%), 54.3 (52.8% to 55.8%) and 55.6 (54.6% to 56.6%) for infants 23–29, 30–31, 32, 33 and 34 weeks’ GA strata, respectively. The subanalysis of 174 infants with paired haematocrits at 0–4 and 4–24 hours showed that for each additional hour after birth, the mean (95% CI) haematocrit increased by 0.2 (0.1% to 0.3%), 0.2 (0.1% to 0.4%) and 0.1 (0.0% to 0.2%) for infants in 23–29, 30–31 and 32 weeks’ GA strata, respectively. The subanalysis showed no change between the paired haematocrits in the 33 and 34 weeks’ GA strata.
Conclusions Our study describes haematocrit in preterm infants who received ≥60 s DCC as standard of care. Haematocrit during the first 0–4 hours in our study is higher than the previously described reference ranges prior to DCC becoming routine clinical practice. The paired second haematocrit at 4–24 hours is higher than haematocrit at 0–4 hours.
- haematology
- paediatric practice
- neonatology
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
What is known about the subject?
Current haematocrit reference ranges for preterm infants were described prior to delayed cord clamping (DCC) becoming routine clinical practice.
Newborn haematocrit increases with each gestational age (GA).
What this study adds?
This study describes haematocrit reference ranges for preterm infants who received at least 60 s of DCC.
There is a greater increase in haematocrit for each GA compared with the previously described references in the pre-DCC population.
Paired sample analysis showed that DCC increases haematocrit from birth to 24 hours after birth in preterm infants 23–32 weeks’ GA.
Preterm infants are born with lower haematocrit than term infants and are at a higher risk for blood transfusion. Studies show delayed cord clamping (DCC) increases placental transfusion and blood volume.1 2 Meta-analyses of more recent studies have shown DCC, compared with early cord clamping (ECC), is associated with higher haemoglobin and haematocrit, higher blood pressure, fewer blood transfusions, reduced intraventricular haemorrhage, and decreased hospital mortality.3 4 A very large population-based study showed reduced severe neurological injury5 in infants 22–28 weeks’ gestational age (GA) receiving DCC.
Hooper et al 6 have rightly suggested that the debate on DCC duration shift away from a time-based approach to a physiology-based approach. Placental transfusion benefit, enhanced by infant ventilation, is the first step in the transfer of sufficient iron essential to optimal brain myelination. Term infants who had their cord clamped after a mean of 172 s compared with a control group who had their cord clamped after a mean of 28 s had higher haematocrit at 48 hours, as well as higher ferritin and evidence of better myelination of the internal capsule and other areas of the brain essential to visual, motor and sensory function/integration at 4 months of age.7 Term infants randomised to 3 min of DCC showed improved fine motor/social function, especially in boys at 4 years of age.8 A randomised controlled trial (RCT) showed preterm infants <33 weeks receiving a mean of 32 s DCC compared with a mean of ECC 6.6 s had better motor function at 18–22 months corrected age.9 In infants <27 weeks, areas of the brain responsible for visual motor integration and fine motor skills are known to be particularly vulnerable to injury, with resultant functional deficits noted 6.5 years later.10 Effective spontaneous ventilation for optimal cardiopulmonary transition in the majority of very preterm infants appears to occur more than a minute or two after birth; thus, timing is relevant to the physiology of transition. Increased DCC duration may be especially important in extremely preterm infants, where umbilical cord milking is less likely to be a viable alternative for placental transfusion benefit.11
The WHO, Neonatal Resuscitation Program, American Congress of Obstetricians and Gynecologists, and other international societies have recommended a minimum duration of DCC of at least 30–60 s to improve preterm neonatal outcomes.12–16
Previously published haematocrit reference ranges based on GA and time after birth were obtained prior to DCC becoming standard of care.17 At our institution, DCC for at least 60 s has been the standard of care since 2011. We have previously shown that implementing a minimum of 60 s DCC in clinical practice increases haematocrit and reduces risk of blood transfusion.18 Haematocrit reference ranges for preterm infants who received DCC as standard of care have not been described; the goal of this study is to present haematocrit for preterm infants <35 weeks’ GA who received at least 60 s DCC.
Methods
Design
This is a single-centre, retrospective observational study.
Patient and public involvement
The study design, analysis and plans to disseminate information from this research were done without patient involvement given this is a retrospective study evaluating DCC and haematocrit which were performed and collected, respectively, as part of our standard of care.
Subjects
Inborn preterm infants <35 weeks’ GA admitted to an American Academy of Pediatrics level 4 neonatal intensive care unit (NICU) in 72 consecutive months (January 2013–December 2018) were eligible for inclusion in the study. Infants were excluded if they (1) received less than 60 s DCC or (2) had no recorded haematocrit within the first 24 hours after birth.
Standardised delivery room and initial preterm infant care
We have established a standardised delivery room management protocol for all preterm infants that includes a minimum of 60 s of DCC since 2011.19 The intended minimum DCC duration was increased to 120 s and 180 s in 2016 and 2018, respectively; the minimum intended DCC duration was not performed when there were maternal or neonatal safety concerns. Umbilical cord milking is discouraged at our institution.
Data collection
Eligible infants were identified from our prospectively maintained NICU database containing maternal and infant demographics as well as neonatal outcomes. Haematocrits were obtained retrospectively from patients’ hospital electronic medical record.
Haematocrit
Complete blood counts (CBCs) were obtained from infants when clinically indicated. Blood samples were obtained from venous, arterial and capillary sources. CBCs between 0–4 and 4–24 hours after birth were used for the study. Time points were chosen because CBCs were routinely obtained in our NICU on admission and on follow-up within 24 hours if indicated. Only the earliest haematocrit was included if more than one was recorded during the time intervals. CBCs from arterial, venous or capillary specimens were measured using Beckman Coulter DxH800 from 2013 to 2014 and using Sysmex XN-3000 from 2015 to 2018. Haematocrits either derived from cord blood samples or obtained after packed red blood cell (RBC) transfusion were excluded.
Data analysis
We used descriptive statistics to describe haematocrit at 0–4 hours after birth for each GA stratum. We summarised the first available haematocrit between 0 and 4 hours according to GA strata: 23–29, 30–31, 32, 33 and 34 weeks’ GA. GA categories were created to provide detailed haematocrit values for each GA group. Younger infants <32 weeks’ GA were combined into shared strata due to limited sample size. Regression models were used to describe the associations between newborn haematocrit and predictor variables including GA, male sex and infant age in hours at the time of haematocrit collection. We used multilevel mixed-effects linear regression with clustering around the mother to account for the presence of multiples (ie, twins, triplets) and clustering around infants with paired haematocrits at 0–4 and 4–24 hours. The multilevel mixed-effects linear regression models used restricted maximum likelihoods and unstructured covariance.
Each regression model was assessed for interactions and multicollinearity. Model residuals were assessed for normality, linearity and homoskedasticity. Outliers were evaluated by running each model with and without infants whose residuals were >2 SD from the mean. Statistical analysis was performed using STATA V.14.
Results
Subjects
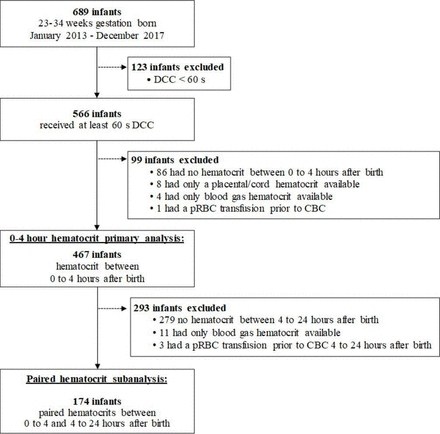
A total of 689 infants were born at <35 weeks’ GA during the study period, of whom 566 (82%) received at least 60 s DCC. Of these 566 infants, 467 (83%) had haematocrit at 0–4 hours (figure 1) used for our primary analysis. For our paired subanalysis, a total of 174 (37%) of the 467 infants were included based on having had an additional haematocrit at 4–24 hours after birth.
Study subject and data selection. CBC, complete blood count; DCC, delayed cord clamping; pRBC, packed red blood cells.
Demographics
Infant and maternal demographics and NICU outcomes are described in table 1. The median GA was 33.3 weeks (range 23.3–34.9) and birth weight was 1910 g (range 400–3241). The median DCC duration was 60 s (range 60–900) and 36% received greater than or equal to 120 s DCC.
Infant and maternal demographics
Haematocrit
Haematocrits at 0–4 hours and 4–24 hours were obtained at a median (IQR) age of 0.9 hours (0.7–1.2) and 13 hours (12–19), respectively. There was no difference in DCC duration or age when CBC specimens were obtained between GA strata. Haematocrit at 0–4 hours increased with each GA stratum (figure 2).
Initial haematocrit (0–4 hours) distribution in gestational age strata. DCC, delayed cord clamping; Hct, haematocrit.
Table 2 shows the associations between newborn haematocrit and predictor variables including GA, male sex and infant age at the time of haematocrit collection. In regression models, GA was positively associated with haematocrit, as demonstrated by the beta coefficient; haematocrit increases by at least 1.2 for each additional week of gestation.
Two-tiered, mixed-effects regression model with clustering around the mother describing the association between preterm newborn Hct at 0–4 hours and predictors including gestational age, male sex and infant age in hours at the time of Hct collection (N=467)
In models 1 and 2, haematocrit was not associated with either male sex or the infant’s age in hours at the time of haematocrit collection.
The subanalysis of the 174 infants with paired samples demonstrated that haematocrits at 4–24 hours were higher than those at 0–4 hours, although only significant for infants <33 weeks’ GA (table 3).
Subanalysis of infants with paired Hct at 0–4 and 4–24 hours after birth showing that Hct increases over 24 hours in infants <33 weeks’ gestation (n=174)
Discussion
Our single-centre study is the largest cohort of preterm infants receiving at least 60 s DCC as standard of care with haematocrits in the first 24 hours after birth. We show that the initial haematocrit in preterm infants receiving at least 60 s DCC increases with GA. At each GA the initial haematocrit (0–4 hours) is higher than that previously published in infants prior to DCC becoming routine clinical practice.17 Individual paired analysis at 0–4 and 4–24 hours allowed us to illustrate a significant increase in the haematocrit during the first 24 hours after birth in those born <33 weeks’ GA.
The mean haematocrit at 0–4 hours in DCC infants in our study was higher for each GA stratum compared with those previously described by Jopling et al.17 The mean haematocrit of infants 28–34 weeks’ GA in our study was 53.5, similar to the haematocrit of 53.8 described by Saigal et al 2 in their 60 s DCC group. A Swiss RCT by Baenziger et al 20 similarly observed a mean haematocrit of 55.56 at 4 hours after birth in 15 infants who received 60–90 s of DCC at 408 m altitude. While Jopling et al 17 showed a 0.64% increase in haematocrit per week of GA between 22 and 40 weeks, we observed 1.2% increase in haematocrit per week between 23 and 34 weeks’ GA. In our study, the incremental placental transfusion benefit in the first minute of DCC is greater for infants with higher GA compared with those with lower GA. Saigal et al 2 showed that both term and preterm infants who received 5 min of DCC increased their blood volume by 47% and 50%, respectively. However, 76% of this transfusion benefit in term infants occurred in the first minute compared with 56% in preterm infants. Both our study and Saigal et al’s2 study suggest that for a given DCC duration, better cardiopulmonary transition in higher GA infants leads to higher placental transfusion benefit.
In our study, there is no difference in haematocrit between 0 and 4 hours, although 75% of our samples were collected within 1.2 hours after birth. Jopling et al 17 previously reported haematocrit loss of 6% in the first 4 hours after birth in preterm infants (22–28 weeks’ GA) but not in infants 29–34 weeks’ GA. This discrepancy in the extremely preterm infants may be explained by practice changes over the last decade, including blood loss prevention.
In our study, within individually paired 0–4 and 4–24 hours haematocrit, analysis showed that haematocrit increased in the first day after birth. Pre-DCC haematocrit reference range study does not show increased haematocrit in the first 24 hours after birth. Our observed increase in haematocrit at 24 hours was not seen in other DCC studies.2 20 Saigal et al 2 showed similar haematocrits obtained from DCC infants at 0.5–4 hours but no increase at 24 hours. Baenziger et al 20 similarly did not observe an increase in haematocrit at 24 hours. The increase in the haematocrit that we observed may be attributable to current clinical practice, including increased DCC duration, less phlebotomy, judicious fluid management and enhanced haemodynamic stability.21 Physiological advantages of DCC include improved cardiac output, renal perfusion and enhanced diuresis, leading to haemoconcentration.
Our study has several limitations. Blood samples were obtained from arterial, venous and capillary sources. It is known that capillary haematocrits are higher than venous haematocrits.22 23 Our single-centre study with 84% antenatal steroid exposure includes better prepared preterm infants whose cardiopulmonary physiology would enhance placental transfusion benefit. We excluded 18% (123/689) of preterm infants who did not receive the intended 60 s duration of DCC due to maternal or neonatal indications. This selection limits our generalisability to all preterm infants <35 weeks’ GA. We also excluded an additional 15% (99/689) of infants primarily due to unavailable haematocrit at 0–4 hours. We suspect most of these excluded infants were clinically stable and early newborn labs may not have been indicated or necessary; thus, they may have been healthier than those included in the study. Less than 37% (174/467) of our cohort had a second haematocrit available, which could cause selection bias. In practice, less stable infants are more likely to have a second recorded haematocrit within 24 hours. This bias is likely to underestimate the increase in haematocrit in the first day after birth and may explain the lack of significant increase in haematocrit in infants 33–34 weeks’ GA.
Our study could have additional utility by providing similar metrics and analysis for haemoglobin. Although haematocrit and haemoglobin are highly correlated and regularly used to assess anaemia, conversion between them is not ideal. Haematocrit is indirectly calculated from the product of the RBC count and mean corpuscular volume, whereas haemoglobin is measured spectrophotometrically. Two different haematology analysers were used during this study, which introduces intermachine variability into our estimates. However, these two analysers have been shown to exhibit good haematocrit agreement and high correlation (r>0.985).24 Furthermore, the results of the regression analysis are unlikely to be biased because the analysers were not preferentially used across GAs, and the same analyser presumably was used for paired haematocrits.
In conclusion, we present a haematocrit reference range for preterm infants who receive at least 60 s DCC as part of standard of care. There is a greater increase in haematocrit for each GA compared with the previously described references in the pre-DCC population.17 Paired sample analysis showed that DCC increases haematocrit from birth to 24 hours after birth in preterm infants 23–32 weeks’ GA.
Acknowledgments
We express gratitude to our patients and families. This work would not be possible without the extreme dedication of SCVMC NICU and Labor and Delivery nursing staff, respiratory therapists, neonatal nurse practitioners, neonatologists, neonatal hospitalists, NICU database team, obstetricians, perinatologists, Stanford University paediatric house staff and SCVMC obstetric house staff, and Santa Clara County First 5 and Valley Medical Center Foundation. We thank MN's clinical research mentors, Hilary Seligman, MD MAS, Thomas Newman, MD MPH, and Isabel E Allen, PhD, for their guidance.
References
Footnotes
Contributors BG, PJ and DS conceptualised the study. BG, PJ, DS, MN and AH designed the study. MN, KG and AH performed the data collection. MN, KG and PJ designed and ensured valid data collection. MN, KG and PJ carried out the data analysis. MN, KG and PJ drafted the initial abstract and the manuscript. All authors critically reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Funding The authors received funding support for data collection for this study from Santa Clara County First 5.
Competing interests None declared.
Patient consent for publication Not required.
Ethics approval This study was approved as a quality improvement project by the Institutional Review Board.
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement Data are available upon reasonable request.