Abstract.
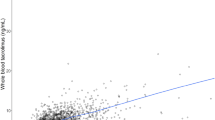
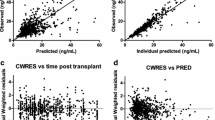
Objective: To characterize the pharmacokinetics of tacrolimus in adult recipients receiving living-donor liver transplantation (LDLT). Methods: Thirty-five patients were given tacrolimus as 18- to 60-h intravenous infusions after surgery, followed by a 4-week course of oral dose therapy (at 0900 hours and 2100 hours). Blood samples were collected daily in the morning (0800 hours) beginning the day after surgery. Whole blood concentration data were evaluated by nonlinear mixed-effect modeling using the program NONMEM and were characterized using a one-compartment model. Results: The clearance (CL, 1 h–1) was related to the grafted hepatic weight, postoperative days (POD), and hepatic and renal dysfunction. Interindividual variabilities in CL, volume of distribution (V), and bioavailability (F) were 57.4%, 39.7%, and 63.0%, respectively, and the correlation between individual CL and F was 0.776. Residual intraindividual variability was 2.9 ng ml–1. Based on the estimated final parameters, a typical recipient of LDLT with grafted hepatic weight of 600 g and normal hepatic and renal function would have a CL of 0.737 l h–1 on POD 0 and 1.14 l h–1 on POD 30, V of 1.52 l kg–1 and F of 6.8%. Conclusions: Nonlinear mixed-effect modeling was useful for analysis of pharmacokinetic characteristics of tacrolimus in LDLT patients. Immediately after surgery, patients receiving LDLT showed a smaller CL value than other transplant patients, and CL value increased with POD within 30 days after surgery. The estimated population pharmacokinetic parameters can be applied for a priori dosage calculations in adult patients with LDLT.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Accepted in revised form: 26 May 2001
Electronic Publication
Rights and permissions
About this article
Cite this article
Fukatsu, .S., Yano, .I., Igarashi, .T. et al. Population pharmacokinetics of tacrolimus in adult recipients receiving living-donor liver transplantation. Eur J Clin Pharmacol 57, 479–484 (2001). https://doi.org/10.1007/s002280100331
Received:
Issue Date:
DOI: https://doi.org/10.1007/s002280100331