Article Text
Statistics from Altmetric.com
What is already known on this topic?
Iron deficiency is one of the most common single-nutrient deficiencies in both developing and developed countries, and peaks in prevalence in early childhood.
For the laboratory assessment of iron status in young children, laboratory reference intervals are not well established and high-quality decision limits based on clinical studies are not available.
What this study hopes to add?
This study presents age- and sex-specific reference intervals for haematological and biochemical tests commonly ordered by clinicians when assessing the iron status of children.
Clinical laboratories may consider adopting the published paediatric reference intervals and age categories, rather than using adult reference intervals.
Future research to develop high quality decision limits based on clinical studies of outcomes is a priority.
Introduction
Laboratory tests play an important role in clinical decision making.1 Clinical laboratories are required to establish or verify ‘reference intervals’ (previously named ‘normal ranges’) for all tests offered by the laboratory.2 3 In conjunction with other health information, clinicians make diagnostic and treatment decisions based on their patients’ laboratory results in the context of the accompanying reference interval. However, clinicians are often unaware of the methods used to establish laboratory reference intervals. Reference intervals are derived from apparently healthy populations and provide two limits (lower and upper limits). The International Federation of Clinical Chemistry (IFCC) established the theory of reference intervals in 1987, and the Clinical and Laboratory Standards Institute (CLSI) provides guidelines for establishing and verifying reference intervals.4 5 These guidelines address issues of selection of the reference subjects, statistical methods including sample size requirements, approaches to grouping results according to categories such as age and sex (termed ‘partitions’) and approaches to the detection and removal of outliers.
Reference intervals differ from ‘decision limits’. Decision limits provide one (or more) cut-points intended to distinguish individuals with disease from those without disease.2 According to a recently published quality hierarchy, decision limits based on clinical outcome studies are considered the highest quality; followed by reference intervals, and cut-off values based on clinicians’ opinions or published professional recommendations.6 An example of a decision limit based on clinical outcome studies is HbA1c for the diagnosis of diabetes.6
It has been recognised that establishing paediatric reference intervals poses specific challenges.3 7 These challenges include the difficulties of obtaining samples from children, and the rapidly changing physiology from the newborn period to late adolescence. A recent systematic review of the current published literature examined the statistical methods used to construct paediatric reference intervals.8 Of the 22 publications identified, only 7 cited and followed the CLSI guidelines.
Iron deficiency is one of the most common single-nutrient deficiencies in both developing and developed countries, peaks in prevalence between 1 and 3 years of age and is associated with poor long-term neurodevelopment which may be irreversible.9 In a report on the diagnosis and prevention of iron deficiency in children under 3 years of age, the American Academy of Pediatrics (AAP) recommends universal screening for anaemia, with measurement of haemoglobin (Hb) concentration and consideration of additional tests to assess iron status, specifically serum ferritin (SF) and C-reactive protein (CRP). Although there are no established decision limits derived from clinical studies of outcomes, the AAP recommends a cut-off value for Hb of <110 g/L and for SF of <12 µg/L. The AAP describes other indicators of iron status including mean corpuscular volume, iron and transferrin. Few studies have examined the reference intervals for laboratory tests of iron status in young children, sample sizes have been small and age partitions wide.10–17
The primary objective of this study was to establish reference intervals for haematological and biochemical tests commonly ordered by clinicians to assess the iron status in young children (10 years and younger) using the CLSI guidelines. A secondary objective was to compare the lower limit of the reference interval with the AAP currently recommended cut-off value for Hb and SF in children 1–3 years of age (age of peak prevalence for iron deficiency).
Methods
Study population
Healthy children, from newborn to 10 years of age, were recruited during a scheduled health supervision visit with their primary care physician. Health supervision visits occur at 2 weeks, 2, 4, 6, 9, 12, 15, 18 months and then annually around the birthday of the child. Recruitment occurred between 2008 and 2016 at paediatric and family medicine primary care practices participating in a practice-based research network called TARGet Kids! (www.targetkids.ca) in Toronto, Canada. The profile of this open longitudinal cohort has been previously described, and children with and without blood samples are similar with respect to demographics and health outcomes.18 Furthermore, the prevalence of iron deficiency is similar to other Canadian studies of this age group.19 Exclusion criteria were: children with associated health conditions affecting growth (eg, failure to thrive, cystic fibrosis), children with any acute or chronic conditions (other than asthma and high-functioning autism), children with severe developmental delay and families who are unable to communicate in English. Parents provided informed consent and completed a questionnaire regarding the child’s age and sex.
Measures obtained by the TARGet Kids! research team include haematological and biochemical tests commonly ordered by clinicians to assess the nutritional status in young children. As a direct benefit of participating in the research, clinicians are provided with results of laboratory tests to be used for clinical purposes. For this study, we analysed data on haematological and biochemical markers of iron status recommended by the AAP; specifically Hb, mean corpuscular volume, three biochemical markers of iron status (SF, iron and transferrin) and CRP. As clinicians commonly request a complete blood count, other haematological tests, including white blood cell count and platelet count, were included in this analysis.
Sample collection and analysis
The research assistants, embedded in the primary care practices, are experienced phlebotomists and collected blood samples in lavender EDTA tubes and in serum separator tubes. Blood samples were refrigerated at the practice sites and transported to the laboratory at Mount Sinai Services (MSS) the same day. MSS provides services to researchers, is accredited and complies with regulatory standards (http://www.mountsinaiservices.com/). At the laboratory, blood samples were analysed fresh within 4–6 hours of collection. For haematology parameters, samples were analysed on the Sysmex XN-9000 Hematology Analyzer (Japan); for chemistry analytes, samples were analysed on the Roche platform (Switzerland) (see online supplementary table 1). The traceability chain used for SF was IS 80/602. The lower level of detection limit for CRP is 0.15 mg/L.
Statistical analysis
For our primary objective, data were analysed following CLSI C28-A3 guidelines and by choosing appropriate statistical methods based on sample size and distribution of the analytes.5 Data were first visually inspected using scatter plots, histograms and probability plots. Outliers were detected and removed using outlier detection techniques described by Horn et al.20 Data were partitioned by sex (male/female). Age partitions were selected based on epidemiological knowledge of the changing prevalence of iron deficiency and overall child growth and development. It is well established that 1–3 years is an age of peak prevalence for iron deficiency, thus we included a specific partition for this age group. Age partitions were as follows: <1 year (infant); >1 year to <3 years (toddler); >3 years to <6 years (preschool/early school age); >6 years to 10 years (school age). Analysis of variance (ANOVA) and pairwise comparisons between adjacent partitions were performed to determine if there was a difference on average laboratory measures across the different age partitions. In preparing the appropriate reference interval method, normality assumption, skewness as well as kurtosis were assessed for each of the laboratory measures per partition. Finally, the parametric or non-parametric method was used to estimate the reference interval as well as 90% CI around the reference intervals after excluding the outliers for each laboratory measure overall and within each partition. The parametric method was used if the normality assumption was met or the sample size in each partition was large; the non-parametric method was used if the normality assumption failed or the sample size was limited (see online supplementary table 2). All p values were two sided and for the statistical analyses, p<0.05 indicated a significantly different result.
For our secondary objective, we further examined Hb and SF in the age partition of children 1–3 years of age, which is the age of peak prevalence for iron deficiency. Using the cut-off values recommended by the AAP (Hb <110 g/L; SF <12 µg/L), we determined the proportion of children misclassified using the lower limit reference interval compared with the cut-off values.
Statistical analysis was performed using SAS V.9.4 system for Windows (Copyright © 2002–2012 SAS Institute) and the R statistical software V.3.2.3 (2015. A language and environment for statistical computing, Vienna, Austria).
Results
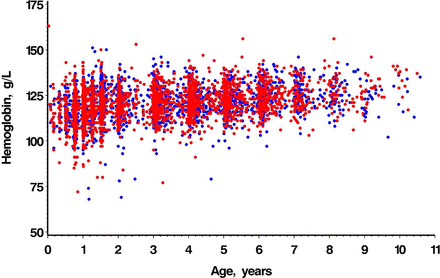
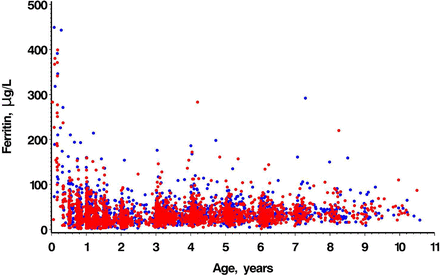
Samples from 2305 male and 2029 female participants (ages 10 days to 10.6 years) were used to calculate age and sex-specific reference intervals for haematological and biochemical tests of iron status. Age and sex are shown in table 1. Scatter plots for Hb and SF are shown in figures 1 and 2. Not all children had blood samples analysed for all analytes, resulting in different sample sizes for each analyte. Some children contributed a blood sample to more than one age group. Age and sex-specific paediatric reference intervals are shown in table 2. ANOVA across age partitions showed statistically significant differences for all analytes; adjacent partition pairwise comparisons of age partitions showed statistically significant differences for all analytes except for CRP (all age comparisons) and transferrin (3> to <6 years compared with ≥6 to 10 years).
Age-dependent scatter plots by sex of haemoglobin concentration (g/L). Red dots=male; blue dots=female.
Age-dependent scatter plots by sex of serum ferritin concentration (µg/L). Red dots=male; blue dots=female.
Characteristics of study population—age and sex
Age and sex-specific reference intervals for analytes (after excluding outliers)
For both Hb and SF in children 1–3 years of age, the lower limit of the reference interval was lower than the currently recommended AAP cut-off value. For Hb, the lower limit of the reference interval was 101 g/L (male) and 102 g/L (female), whereas the currently recommended AAP cut-off value is 110 g/L; the proportion misclassified (underestimated) was 10.78% for men and 9.76% for women. For SF, the lower limit of the reference interval was 6 µg/L (male) and 7 µg/L (female), whereas the currently recommended AAP cut-off value is 12 µg/L; the proportion misclassified (underestimated) was 10.19% for men and 10.97% for women.
Discussion
We have established age and sex-specific reference intervals for several haematological and biochemical tests commonly ordered by clinicians when assessing the iron status of children. Age partitions showed statistically significant differences for most analytes, suggesting that laboratories should use these age categories. Furthermore, we found that approximately 10% of children 1–3 years of age would be misclassified as having healthy iron status (no disease) according to measures of Hb and SF, if the lower limit of the reference intervals was used rather than the cut-off values currently recommended by the AAP.
In recognition of the critical gaps in paediatric reference intervals, the IFCC Task Force on Paediatric Laboratory Medicine launched an initiative to coordinate international efforts to fill this gap, representing groups from several countries.21 One group, the Canadian Laboratory Initiative on Pediatric Reference Intervals (CALIPER), has published several analyses that include reference intervals for several analytes relevant to the assessment of iron status in young children (see table 3); reference intervals are also accessible on their database website.10–15 22 However, data from CALIPER remain limited due to small sample sizes, especially for very young children, when iron deficiency peaks in prevalence. For example, Bailey et al presented reference intervals for SF based on 126 children 1–5 years of age.12 Previous publications on the prevalence of iron deficiency in young children used data from the nationally representative US National Health and Nutrition Examination Survey (NHANES). These included a large number of laboratory tests for Hb, SF and other biochemical analytes.23–25 However, NHANES did not establish reference intervals according to the CLSI guidelines.
CALIPER published reference intervals for several analytes relevant to the assessment of iron status in children
Given the challenges of developing reference intervals, it is difficult for each laboratory to develop their own reference intervals for children. As a result, clinicians are often provided with reference intervals for adults only which may lead to substantial misclassification, and possible overdiagnosis or underdiagnosis. Several prerequisites have been described in order for clinical laboratories to adopt common reference intervals.3 26 These include the following: the population served by the clinical laboratory is similar to that from which the reference interval was developed; the clinical laboratory uses methods that the manufacturers certify produce results that are traceable to the reference measurement system for a specific analyte; precision of the methods is within acceptable targets. It is also recommended that the clinical laboratory validate the reference interval in a small sample of their own population.3 27
Reference intervals have inherent limitations in their application to clinical decision making. By their nature, reference intervals describe the characteristics of the referent population, which, while assumed to be healthy, may include asymptomatic individuals. Decision limits provide clinicians with the dichotomous information (disease/no disease) they seek for clinical decision making. In our study, we found wide reference intervals for SF, for example, which suggests that many children with asymptomatic iron deficiency were included in the referent population. Therefore, reference intervals may not adequately inform clinical decision making for the laboratory assessment of iron status in young children. Laboratories may also provide decision limits when available and cut-off values from published recommendations from professional organisations.6
Strengths of our study include a large sample of healthy children recruited from community settings during a health supervision visit with their primary care physician, and adherence to the CLSI guidelines. For the young age group at highest risk for iron deficiency (1–3 years), our sample size is substantially larger than previously published reference intervals.
Our study was limited to young children under 10 years of age. To fill the gaps in paediatric reference intervals, other initiatives, such as CALIPER, have large sample sizes for children older than 10 years. Furthermore, we have not addressed cut-off values for children 3–10 years of age.
For our secondary objective, our study findings are limited by the low quality of evidence used to establish the currently recommended AAP cut-off value, especially for SF. The currently recommended cut-off value of 12 µg/L for SF is not based on a clinical outcome (as required for a high-quality decision limit), but rather based on the fifth percentile of the distribution of SF in a population presumed to be healthy.23 In a recent analysis examining the relationship between Hb and SF, we identified a SF value of 23.7 µg/L corresponding to the level at which Hb concentration was maximised.28 If the true decision limit for SF is indeed as high as 23.7 µg/L (rather than the currently recommended cut-off value of 12 µg/L), the proportion of children misclassified using the lower limit of the reference interval would be substantially higher.
Conclusion
We have established reference intervals for haematological and biochemical tests commonly ordered by clinicians to assess the iron status in young children under 10 years of age using the CLSI guidelines. As clinical laboratories are required to provide reference intervals for all tests offered, they may consider adopting published paediatric reference intervals. We have described the methodological approaches to establishing reference intervals and decision limits to better inform clinicians of their benefits and limitations as applied to clinical decision making. In the absence of decision limits based on clinical studies of outcomes (highest quality of evidence), we have described the currently recommended cut-off values for Hb and SF in the assessment of children 1–3 years of age and have identified the potential for misclassification when using reference intervals alone. Future research should focus on establishing decision limits for the laboratory assessment of iron status based on clinical outcomes.
Acknowledgments
We thank all of the participating children and families for their time and involvement in the TARGet Kids! primary care practice-based research network and all practice site physicians, research staff, collaborating investigators, trainees, methodologists, biostatisticians, data management personnel, laboratory management personnel and advisory committee members (details may be found on our website: www.targetkids.ca).
References
Footnotes
Contributors PCP conceptualised and designed the study, designed the data collection instruments, interpreted the data, drafted the manuscript, critically revised and reviewed the manuscript for important intellectual content, and approved the final manuscript. JH conceptualised and designed the study, analysed and interpreted the data, critically revised and reviewed the manuscript for important intellectual content, and approved the final manuscript. CMB and KA conceptualised and designed the study, interpreted the data, critically reviewed the manuscript for important intellectual content and approved the final version of the manuscript. EGA performed the statistical analysis, interpreted the data, critically reviewed the manuscript for important intellectual content and approved the final version of the manuscript. JLM and CSB designed the data collection instruments, supervised the data collection, critically reviewed the manuscript for important intellectual content and approved the final version of the manuscript. AA, VH and KA interpreted the data, critically reviewed the manuscript for important intellectual content and approved the final version of the manuscript.
Funding Funding to support TARGet Kids! was provided by multiple sources including the Canadian Institutes for Health Research (CIHR), namely the Institute of Human Development, Child and Youth Health and the Institute of Nutrition, Metabolism and Diabetes, as well as the St. Michael's Hospital Foundation. The Pediatric Outcomes Research Team is supported by a grant from The Hospital for Sick Children Foundation. Funding agencies had no role in the design, collection, analyses or interpretation of the results of this study or in the preparation, review or approval of the manuscript.
Competing interests None declared.
Patient consent Obtained from the parents/guardian.
Ethics approval Hospital for Sick Children Research Ethics Board and the St. Michael's Hospital Research Ethics Board.
Provenance and peer review Not commissioned; externally peer reviewed.