Article Text
Abstract
Background Hypoxaemia is a common and potentially fatal complication of many childhood, newborn and maternal conditions but often not well recognised or managed in settings where resources are limited. Oxygen itself is often inaccessible due to cost or logistics. This paper describes implementation of oxygen systems in Lao district hospitals, clinical outcomes after 24 months and equipment outcomes after 40 months postimplementation.
Methods A prospective field trial was conducted in 20 district hospitals, including 10 intervention hospitals that received oxygen concentrators and 10 control hospitals. Equipment outcomes were evaluated at baseline, 12, 24 and 40 months. Clinical outcomes of children under 5 years of age with pneumonia were evaluated using a before-and-after controlled study design with information retrospectively collected from medical records.
Results Fourteen (37%), 7 (18%) and 12 (34%) of 38 concentrators required repair at 12, 24 and 40 months, respectively. The proportion of children discharged well increased in intervention (90% (641/712) to 95.2% (658/691)) and control hospitals (87.1% (621/713) to 92.1% (588/606)). In intervention hospitals, case fatality rates for childhood pneumonia fell from 2.7% (19/712) preintervention to 0.80% (6/691) postintervention with no change in control hospitals (1.7% (12/713) preintervention and 2.3% (14/606) postintervention).
Conclusion Medium-term sustainability of oxygen concentrators in hospitals accompanied by reduced case fatality for childhood pneumonia has been demonstrated in Lao PDR. Significant local engineering capacity to address multiple causes of equipment malfunction was critical. The ongoing requirements and fragile structures within the health system remain major risks to long-term sustainability.
- general paediatrics
- health services research
- outcomes research
- tropical paediatrics
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Statistics from Altmetric.com
What is already known on this topic?
Hypoxaemia is a potentially fatal complication of many common childhood, neonatal and adult health problems.
Hypoxaemia is often not well recognised or treated in many low-resource settings, and oxygen is often inaccessible.
Systematic use of pulse oximetry for detecting hypoxaemia and availability of reliable oxygen sources can reduce death rates from pneumonia in children.
What this study hopes to add?
Represents one of the most comprehensive evaluations of implementation of oxygen concentrator systems in district hospitals.
Improved outcomes for childhood pneumonia in hospitals that received oxygen concentrator systems that included equipment, engineering and clinical training.
The potential maintenance and engineering burden of concentrators on already fragile health systems.
Introduction
Hypoxaemia, or insufficient oxygen levels in the blood, is a potentially fatal complication of many common causes of mortality in children, newborns and adults.1 It is estimated that at least 13% of children with WHO-defined pneumonia requiring hospitalisation have hypoxaemia, with wide geographical variation.2 3 Despite its importance, hypoxaemia is often not well recognised or managed in settings where resources are limited.4 5 Evidence from previous studies suggests systematic use of pulse oximetry for detecting hypoxaemia and availability of reliable oxygen sources can reduce death rates from pneumonia in children by up to 35%.6
In low and middle-income countries, oxygen can represent a significant hospital expense. In countries with a user-pays health system oxygen may be available, but the cost to the patient can limit use or precipitate early discharge. High costs of oxygen partly reflect logistical difficulties in transporting oxygen, which also lead to intermittent availability of oxygen cylinders. High costs to patients and lack of access to oxygen potentially undermine community confidence in hospitals and are a major impediment to universal access to healthcare.
Oxygen concentrators—small machines that remove nitrogen from atmospheric air to generate 85%–95% oxygen—are one potential solution.7 They have been found to be feasible for delivery of oxygen in low-income countries, although there is variability in performance.8–10 For concentrator systems to be sustainable, they require a continuous and reliable power supply, a system for monitoring, maintenance and repair, as well as clinical staff trained in their use.7
The Lao Oxygen Therapy Pilot Project was established in recognition of the complex issues, and multiple barriers, relating to oxygen supply in Lao PDR. The aims of the project were to implement an affordable oxygen system in district hospitals and to evaluate its feasibility, sustainability and impact on patient care and outcomes. This paper describes the implementation approach, clinical outcomes at 24 months and equipment outcomes at 40 months postimplementation.
Methods
Hospital selection
Intervention hospitals were selected based on criteria including availability of reliable electricity, absent or limited oxygen supply, remote geographical location and anticipated admission numbers. Two northern and three southern provinces were included for different climates and disease patterns. Randomisation was not feasible given the selection criteria; however, control hospitals were recruited and selected from the same provinces based on similarity to intervention hospitals. One provincial hospital was included as a control as it was equivalent to the larger district hospitals.
Implementation of oxygen systems
The project consisted of four phases; a situational assessment, establishment of the oxygen team and partners, systematic implementation of the oxygen systems and evaluation of clinical use of oxygen, equipment use and maintenance requirements, clinical outcomes and patient costs. (figure 1) The Lao Oxygen team included staff from the Lao Ministry of Health, local adult and paediatric intensive care physicians, provincial paediatricians, international paediatricians, an international biomedical engineer and staff from the Lao Country Office of the WHO. An agreement was made that oxygen delivered from project concentrators would be free to patients, removing any financial impediments to access.
Graphical representation of study design and time points.
Implementation of oxygen systems in selected hospitals occurred from September to November 2011. All 10 intervention hospitals were visited by the installation team to check existing resources, install the oxygen system and provide biomedical engineering training on its use and maintenance. This was followed closely by the clinical training team, consisting of clinicians from the Lao Oxygen team.
The clinical training team delivered Lao-specific training materials based on WHO guidelines for oxygen11 and hospital care of children12 which included videos, guidelines and case-based teaching sessions. On completion of training, each participant underwent an observational assessment based on recognition and treatment of hypoxia, as well as oximeter use. Control hospitals were given pulse oximeters but did not receive any training on their use.
Equipment
The VisionAire Oxygen Concentrator (AirSep, Buffalo, New York, USA) was chosen because of several unique features that were considered to be appropriate for Laos.7 Unlike other concentrators, the VisionAire has no external filter that typically require regular maintenance and replacement. Removing this maintenance step was felt to be advantageous. The concentrator was specified to deliver 5 L per minute of oxygen in the concentration range of 82%–95% at 21°C degrees and operate at temperatures between 5°C and 35°C degrees with relative humidity up to 95%. Oxygen analysers (Hudson RCI, http://hudsonrci.com/) were provided to monitor the performance of concentrators.
A mains-powered, table-top oximeter was chosen from a major German manufacturer (Bitmos). It was selected to minimise risk of equipment loss and because the software rejects movement signals facilitating accurate use in children. Oximeter sensors with a guaranteed life of 1 year were sourced from Carril Instruments, Barcelona.
Evaluation design
A prospective field trial was conducted in 20 district hospitals, including 10 intervention hospitals that received oxygen concentrator systems and 10 control hospitals. This included a before-and-after controlled study of the outcomes of children less than 5 years of age with an admission diagnosis of pneumonia.
Equipment outcomes
Prospective evaluation of equipment outcomes was undertaken with data drawn from a number of sources including the field notes from meetings and the installation of oxygen systems, hospital reports and site visits to both control and intervention hospitals at baseline, 3, 12 and 24 months. Hospital equipment checklists and surveys were completed at both intervention and control hospitals at each visit. Data collected at these times included routine hospital admission data, hours of oxygen use as read from the concentrator display and the availability and function of equipment. An additional visit to intervention hospitals was conducted 40 months after implementation, more than 1 year after the formal project ended, to understand the sustainability beyond the project timeframe.
Clinical outcomes
Clinical data were collected in both intervention and control hospitals before and after the implementation. Admission books were retrospectively reviewed at each hospital preintervention (2011) and postintervention (2014) to identify children under 5 years of age who were admitted with pneumonia or a lower respiratory tract infection. Data were collected by a paediatric resident or paediatrician familiar with WHO classification of pneumonia and trained to identify signs or symptoms of pneumonia from records. Patients with diagnoses of ‘pneumonia’, ‘cough’, ‘respiratory distress’, ‘bronchitis’, ‘respiratory infection’ and ‘bronchiolitis’ were included in the initial identification of patients but excluded later if (1) the medical record had been lost or (2) the clinical description in the medical record was inconsistent with the WHO-defined pneumonia.
Preintervention we aimed to identify 100 cases of pneumonia/lower respiratory tract infection from each hospital. If 100 cases were not found, data collection continued back to a maximum of 5 years (2007). Postintervention data were collected for cases admitted between January 2012 and December 2013.
A standardised data abstraction form was used to collect patient demographics, diagnosis, pneumonia severity, documentation of SpO2, oxygen therapy and patient outcome. Clinical signs and severity of pneumonia documented by the admitting doctor were reviewed by data collectors and investigators (MM/AZG) and if clinical signs indicated a different severity, then pneumonia severity was reclassified based on available data.
The primary outcome measure was the proportion of patients under 5 years of age with pneumonia who stayed in hospital to complete a course of treatment and were discharged well, with no signs of ongoing illness documented in the medical record. This was based on our hypothesis that improving access to oxygen would facilitate treatment completion and reduce early discharge.
Secondary outcomes included: the proportion of children under 5 years of age with pneumonia who were discharged unwell with ongoing signs of illness; the proportion of children who died; and the proportion of children in whom peripheral oxygen saturation (SpO2)and oxygen therapy was documented.
Statistical analysis
The sample size calculation for clinical outcome data was based on estimates from our baseline assessment that 5% of children with pneumonia are discharged while still unwell, or die in hospital, and an expectation that the oxygen intervention might halve this proportion. A sample size of 906 cases was required in both the intervention and control arms. For other outcome measures, such as the proportion of patients monitored, this estimated sample size was likely to be conservative due to higher prevalence of the measured outcome or larger expected effect size.
Data were entered and stored securely in EpiData databases. Analysis was done using SPSS software (version 16.0). Descriptive analysis of quantitative data was performed with categorical variables described in terms of numbers and percentages and numerical variables described according to their median and range. Tests of proportions were performed on categorical variables preintervention and postintervention using χ2.
Ethics
Ethical clearance was granted by the Lao National Ethics Committee for Health Research and the Human Research Ethics Committee of The University of Melbourne in 2011. Written consent was obtained from each participating hospital.
Results
Equipment outcomes
Control and intervention cohorts had similar bed and staff numbers as well as oxygen costs at baseline, while annual admission numbers varied (table 1). The median costs of provision of oxygen to a patient was approximately 200 000 Kip (US$25) per cylinder.
Characteristics of study hospitals in 10 intervention and 10 control hospitals in the Lao Oxygen Project at baseline
In the first year following installation 37% (14/38) of concentrators experienced a fault, with a higher proportion in southern compared with northern provinces. (table 2) Seven concentrators (18%) needed sieve beds replaced, and four concentrators (11%) required a new compressor, with other repairs being relatively minor. Following repair, 92% (35/38) of the concentrators were functional. The limiting factor was the quantity of spare parts rather than local engineering capacity. Given the equipment repair requirements at this time a request was made to the manufacturer to replace 10 concentrators with an updated model with improved airflow and compressors and to replenish spare parts. Nine were distributed to hospitals with concentrators requiring major repairs, or those in which problems were anticipated, with one kept in reserve in the central equipment stores.
Concentrator repair and maintenance at 12, 24 and 40 months postimplementation of oxygen systems in 10 intervention hospitals and hours of concentrator use in each time interval
At 24 months, 18% (7/38) of concentrators required repair including one new model machine, and all but one were repaired. Forty months postimplementation, one-third of concentrators (12/32) required repair, of which 10 (83%) were original machines (table 2). It is useful to understand the cumulative failure rate of concentrators over time; however, due to concentrators being replaced or removed from operation over time, this was difficult. Instead cumulative failure rates can be expressed as a proportion of the number of ‘concentrator years of operation’ (ie, number of failed concentrators/total number of concentrators in field × number of years). For the original models, the proportion of machines failing was 0.37, 0.30 and 0.28 at 12, 24 and 40 months, respectively, compared with 0.11 and 0.14 for new model concentrators at 24 and 40 months, respectively.
Concentrators were used for 13 465 hours and 17 050 hours in the first and second years of the project respectively, with an overall total of around 30 000 hours. Accurate measurement of hours past 24 months was difficult due to movement of equipment between hospitals and concentrators taken out of service. There were no adverse incidents, such as a fire, from the use of concentrators during the study period.
All oximeters and sensors were functional 1 year after implementation. After 2 years, 9 of the 10 oximeters in intervention hospitals were still functioning and the majority of the oximeter sensors. By 40 months, 70% of oximeters remained functional, while the proportion of functional sensors varied widely between hospitals from 100% (H2P3) to none (H1P2).
Clinical outcomes
A total of 2912 standardised data abstraction forms were collected from intervention and control hospitals, of which 2777 remained after excluding children greater than 5 years of age and 2722 after excluding other diagnoses. Table 3 outlines the characteristics of childhood pneumonia cases in the preimplementation and postimplementation era from control and intervention hospitals. Patient numbers, age, length of stay and pneumonia severity were similar between hospital cohorts both preintervention and postintervention. There was variation in the proportion of patients contributed by different study hospitals preintervention and postintervention. There was a reduction in the proportion of severe/very severe pneumonia cases in the postintervention era, but the proportions were comparable between intervention and control hospital cohorts.
Admission numbers and characteristics of children under 5 years of age with pneumonia whose medical records were reviewed in preimplementation and postimplementation of oxygen systems in Lao district hospitals
Preintervention, between group analysis demonstrated a small difference between control and intervention hospitals in the proportion of patients discharged well, with no difference in the proportion discharged with ongoing signs of illness, or who died in hospital, both overall and in the most severe pneumonia cases (table 4). Postintervention, the proportion of all patients with pneumonia discharged home well improved in both the control and intervention hospitals. In patients with severe or very severe pneumonia, the proportional increase in patients discharged well was greater in the intervention compared with control hospital (table 4).
Recorded outcomes of children under 5 years of age admitted with pneumonia to study hospitals preimplementation and postimplementation of oxygen systems
There was a corresponding reduction in the proportion of pneumonia patients discharged home unwell with documented signs of ongoing illness in both control (8.1% (58/713) preintervention and 3.3% (20/606) postintervention, RR 0.41 (95% CI 0.25 to 0.67)) and intervention hospitals (6.2% (44/712) preintervention and 1.9% (13/691) postintervention, RR 0.30 (95% CI 0.16 to 0.56)) (table 4). In the preintervention era, approximately 70% of these patients had documentation of discharge against medical advice, and this fell to 25% of cases postintervention accounting for most of change in patient numbers.
In intervention hospitals, case fatality rates for childhood pneumonia fell from 2.7% (19/712) preintervention to 0.80% (6/691) postintervention (RR 0.33, 95% CI 0.13 to 0.81) with no change in control hospitals (1.7% (12/713) and 2.3% (14/606), RR 1.37, 95% CI 0.64 to 2.95) (table 4). The same trend was seen for the most severe pneumonia cases (table 4).
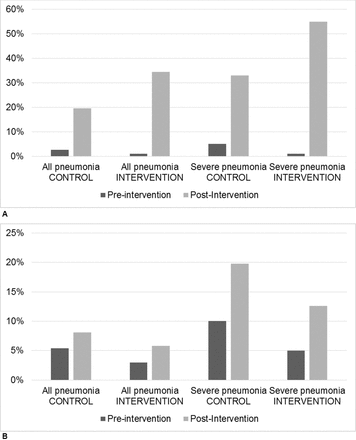
Improved processes of care were seen in both intervention and control hospitals including increased documentation of SpO2 in medical records, with a larger proportional increase in intervention hospitals (figure 2a). In both cohorts, SpO2 was more frequently documented in the more severe cases. Less improvement was seen in the proportion of children having oxygen therapy and/or oxygen flow rates documented, with minimal difference between hospitals (figure 2b).
Percentage of cases of pneumonia in children less than 5 years of age in control and intervention hospitals in which (A) SpO2 and (B) oxygen therapy were documented in the medical records, preintervention and postintervention.
Discussion
In 2010, the Lao Ministry of Health identified the lack of affordable oxygen therapy as a major health system challenge and responded with a pilot project to deliver affordable oxygen concentrator systems to district hospitals. The project built on lessons learnt in other settings and an increasing knowledge basis of the types of concentrators and equipment that may best suit different environments.9 10 There is evidence of its impact on care and outcomes, with a reduction in case fatality rates for childhood pneumonia in intervention but not control hospitals. However, there are also ongoing challenges, and the engineering investment and health system support required to achieve these outcomes must be recognised.
The project was embedded in the Ministry of Health and implementation decisions were made by a team supported by international partners. Lao clinicians and engineers received hands-on training aimed at building local. The local engineering capacity that was developed and sustained was evident by their ability to address substantial maintenance and repair requirements for concentrators. Despite this, many oxygen concentrators failed, and the impact of the maintenance requirements on feasibility and sustainability of concentrator systems should not be underestimated. Failure rates of the newer model replacement concentrators appeared to be less. Concentrator faults were reported either through the normal health system communication channels or through project evaluation activities. The majority of the equipment issues were only identified through the latter. In the absence of an ‘oxygen project’, there remains a major risk that equipment falters and is abandoned.
Identifying reasons for equipment failure is essential. The high proportion of the original model concentrators requiring repair likely reflects poor suitability to the environmental conditions. The majority of major faults occurred in southern provinces where temperatures of 40°C degrees are combined with humidity and dust. Laboratory testing of concentrators that occurred after the project began support our observations that the original machines do not function optimally in these conditions. Investigations demonstrated that the AirSep VisionAire was less reliable at high temperature and simultaneous high humidity than the earlier model AirSep Elite.10 This was attributed to an inadequate internal cooling system in the VisionAire that leads to higher running temperatures and in turn failure of the compressor and sieve beds as we saw in Laos. Despite modifications, the manufacturer no longer recommends the VisionAire for use in tropical conditions.
Environmental conditions will not be the sole explanation for equipment failures. Hospital differences are apparent, even in geographically similar locations. The marked discrepancies in functioning oximeter sensors in some hospitals compared with others suggests local factors such as attention to maintenance and loss are also important. Further exploration of high-performing and low-performing case examples may provide insight into hospital-level influences on the effectiveness of oxygen concentrator systems and medical equipment in general.
The total of 30 000 hours over the first 2 years of the project represented only 5% of the potential 665 760 hours the machines were available for use during this time. This in part will be due to relatively low admission numbers of Lao district hospitals. It is may also reflect underuse of oxygen in cases in which it may have been indicated, or equipment downtime from faults.
Despite these problems, this study provides evidence of improved oxygen use and clinical outcomes for children under 5 years of age with pneumonia. Reduction in the proportion of children with pneumonia who were discharged unwell before completing treatment was seen in both intervention and control hospitals, with a corresponding increase in the proportion discharged well. In control hospitals, this may reflect a number of factors including a Hawthorn effect from participation in the study, with increased awareness of the importance of oxygen therapy. It may also reflect access to oximetry to determine completion of treatment, or other secular trends such as improved financial circumstances of the community. Finally, case severity impacts on outcomes and in both the control and intervention groups, there was a reduced proportion of severe and very severe pneumonia in the postintervention era. However, these changes were comparable between control and intervention hospitals—they do not explain the reduction in the proportion of patients discharged unwell, which was observed among the most severe pneumonia cases, nor do they explain the reduction in case fatality observed only in intervention hospitals. Therefore, although there are many confounding factors to consider, the reduction in pneumonia case fatality documented in intervention hospitals seems likely to be explained, at least in part, by access to improved oxygen systems as seen in previous studies of oxygen implementation.6
Improvements in documentation of SpO2 and oxygen delivery were also seen with a greater degree of improvement in the intervention group. For pulse oximetry, this may reflect improved availability of oximeters to control hospitals, compared with more intense training in their use at intervention hospitals. Perceived relevance of documenting SpO2 is also likely to be a factor as suggested by higher levels of documentation in more severe cases. Gaps remain between actual and ideal practice. A range of other factors in individual hospitals are likely to contribute to the lack uptake of recommended practices, including the lack of a specific location on monitoring charts to document oximetry and oxygen, and established hospital practices for patient monitoring which require a broader cultural change.
There are limitations in the study design. The inclusion of a control group of hospitals improves on previous assessments of oxygen systems. However, the inability to allocate oxygen systems using an experimental design still limits interpretations of outcomes. Improved clinical outcomes and mortality rates may have been due to confounding factors, as discussed previously, but we are unaware of other interventions that may have affected hospitals differentially. The accuracy and detail of medical records limited our ability to accurately determine pneumonia severity or patient outcomes in some instances. Medical records represented those we could locate; we cannot be sure how many children were admitted with pneumonia in the 20 hospitals in each of the two time periods. Variation between hospitals is evident and, similar to equipment outcomes, changes in clinical practice may be influenced by hospital characteristics that we were not able to explore in detail. For some hospitals, the project provided the first reliable source of hospital oxygen, while for others, it only removed the financial barrier.
Delivery of an affordable, feasible and sustainable oxygen system to prevent hypoxaemia and reduce mortality is a critical intervention in resource-limited settings. It requires a multifaceted approach including robust oxygen equipment, well supported local clinical and engineering capacity and ongoing support for the clinical use of oxygen. However, oxygen concentrator systems can create significant ongoing maintenance demands, which already fragile health systems in low resource settings must be able to support to achieve long-term sustainability, unless more robust equipment solutions can be found. There would be clear benefit from a more detailed understanding of contextual factors influencing equipment longevity and clinical outcomes in order for future oxygen programme to have maximal impact.
References
Footnotes
Contributors AZG contributed to study design, developed the clinical training materials, assisted with the implementation of oxygen systems, supervised data collection and training of data collectors, analysed the data and was responsible for the first draft of the manuscript. MM contributed to supervision of data collection in the postimplementation era, entered, cleaned and analysed data and contributed to both the first draft and subsequent revisions of the manuscript. TD contributed to study design, planning of implementation and contributed to interpretation of results and revision of the manuscript. DP contributed to study design, provided technical expertise into the engineering aspects of the programme, supervised implementation of equipment and contributed to revision of the manuscript. CW contributed to study design, equipment procurement and implementation of oxygen systems as well as revision of the manuscript. MS contributed to study design, in particular hospital selection and liaison, implementation of oxygen systems, monitoring of equipment in the field and reviewed the manuscript. KS contributed to study design, clinical training in oxygen use, data collection from medical records and supervised other data collectors and reviewed the manuscript. BP contributed to study design, clinical training in oxygen use, data collection from medical records and supervised other data collectors and reviewed the manuscript. KD contributed to study design, clinical training in oxygen use and supervision of data collectors and reviewed the manuscript.
Funding This work was supported by the Japanese Embassy Lao PDR through the WHO Office, who procured the equipment for the project. Additional funding for evaluation came via AusAID funding to the Centre for International Child Health, The University of Melbourne. We thank the manufacturer Airsep for responding generously to our request for replacement machines and spare parts in the second year of the project. The funding sources had no role in the design of the study or the decision to publish this article.
Competing interests None declared.
Ethics approval Human Research Ethics Committee of The University of Melbourne and Lao National Ethics Committee for Health Research.
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement On request we are happy to share the data pertaining to this study. Requests should be made by email to the corresponding author.