Article Text
Abstract
Background Early onset sepsis (EOS) is defined as onset of sepsis within 72 hours after birth. Leucocyte-endothelial interactions play a pivotal part in EOS pathophysiology. Endothelial cell adhesion molecules (CAMs) orchestrate these interactions and their soluble isoforms (sCAMs) are released into the vasculature by enzymes called sheddases.
Purpose This study was undertaken to explore further the pathophysiology of EOS and to investigate the potential of sCAM and their sheddases as potential biomarkers for EOS.
Methods Stored serum aliquots were used from 71 Surinamese newborns suspected of EOS and 20 healthy newborns from an earlier study. Serum had been collected within 72 hours after birth and six (8.6%) newborns had a positive blood culture with gram-negative pathogens. Concentrations of sCAMs sP-selectin, sE-selectin, soluble vascular cell adhesion molecule-1 , intercellular adhesion molecule-1 and platelet and endothelial cell adhesion molecule-1, sheddases matrix metalloproteinase-9 (MMP-9) and neutrophil elastase (NE) and sheddase antagonist tissue-inhibitor of metalloproteinases-1 (TIMP-1) were measured simultaneously with Luminex and ELISA.
Results MMP-9 and TIMP-1 levels were measured in serum of n=91 newborns and sCAMs and NE levels in serum of n=80 newborns, respectively. We found no differences in median concentrations of sCAMs, MMP-9 and TIMP-1 or NE between blood culture positive EOS, blood culture negative EOS and control groups at start of antibiotic treatment.
Conclusions Our data indicate that serum concentrations of sCAMs and their sheddases have no clinical utility as biomarkers for EOS.
Trial registration number NCT02486783. Results
- newborns
- early onset sepsis
- adhesion molecules
- shedding
- Suriname
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
What is already known on this topic?
Recently, we established an association of the Angiopoietin (Ang)-1/Ang-2 disbalance with blood culture positive early onset sepsis (EOS) in newborns.
The relationship between this Ang-1/Ang-2 disbalance and serum levels of soluble isoforms of cell adhesion molecules (sCAMs) and their sheddases is unclear.
What this study hopes to add?
The Ang-1/Ang-2 disbalance in blood culture positive EOS is not paralleled by increased levels of sCAMs and their sheddases.
Levels of sCAMs and their sheddases are high after birth and do not discriminate EOS from healthy newborns.
Introduction
Early onset sepsis (EOS) in newborns within 72 hours after birth remains a clinical challenge with high morbidity and mortality.1–3 The majority of global neonatal deaths due to EOS occur in developing countries.4 The diagnosis of EOS is complicated, resulting in late recognition or overtreatment of newborns with antibiotics. These dilemmas arise because the pathophysiology of EOS is poorly understood.
A hallmark of sepsis pathophysiology is endothelial cell activation followed by leucocyte recruitment into tissues.5 The current model describes the occurrence of a shift in balance in Tie2 receptor ligands Angiopoietin (Ang)-1 and Ang-2 affecting endothelial integrity and increased expression of endothelial cell adhesion molecules (CAMs), in particular P-selectin, E-selectin, vascular cell adhesion molecule (VCAM-1) and intercellular adhesion molecule (ICAM-1) to facilitate this recruitment.6 7 These endothelial CAMs orchestrate leucocyte rolling on, adhesion to and diapedesis across the endothelium.7 8 Also, platelet and endothelial cell adhesion molecule (PECAM-1), expressed at endothelial cell junctions has a function in facilitating paracellular transmigration of leucocytes across the endothelium.9 After intravenous administration of endotoxin in healthy adults as a sepsis model, peak concentrations of Ang-2 prelude the release of soluble isoforms of cell adhesion molecules (sCAMs) into the systemic circulation.10 Endothelial CAMs are released through ectodomain shedding by enzymes called sheddases, in particular matrix metalloproteinase-9 (MMP-9) and neutrophil elastase (NE), released from granules in neutrophils.7 11 Both MMP-9 and NE prepare the extracellular matrix for transmigration of leucocytes into inflammatory sites.12 MMP-9 activity is balanced by sheddase antagonist tissue-inhibitor of metalloproteinases-1 (TIMP-1).12–14
Recently, we showed in a cohort of near term and term Surinamese newborns that a systemic disbalance in Ang-2/Ang-1 concentrations was associated with blood culture positive EOS.15 This study was undertaken to examine if this disbalance is paralleled by increased serum concentrations of sCAMs and sheddases in this cohort of newborns with EOS to explore further the pathophysiology of EOS. We hypothesised that sCAM and sheddases concentrations measured at start of antibiotic treatment for suspected EOS are higher in newborns with blood culture positive EOS than in healthy controls.
Materials and methods
Study design, subjects and clinical protocol
For this study, we used a Surinamese cohort of 20 healthy newborns and 71 newborns with suspected EOS from an earlier reported study.15 All newborns were included after admission between 1 April 2015 and 31 May 2016 to the neonatal care facility of Academic Pediatric Center Suriname at the Academic Hospital Paramaribo in Suriname. Included were newborns with a gestational age equal to or above 34 weeks in whom antibiotics were started within the first 72 hours of life for suspected EOS. Suspicion of EOS was based on the attending physicians decision to start antibiotic treatment. Informed consent was obtained from at least one parent for the use of residual serum and clinical information. The study protocol was made available on clinicaltrials.gov (NCT02486783) and was approved by the Surinamese Medical Ethical Board (VG-021-14A) including permission of one parent.
The management of these patients was described before.15 In short, healthy control newborns and newborns suspected of EOS were included at start of antibiotic treatment within 72 hours after birth. At start of antibiotic treatment, blood was collected for separation and storage of serum. Controls were newborns without signs of infection receiving blood draws for hyperbilirubinaemia (n=20). Newborns with suspected EOS receiving treatment with intravenous antibiotics were divided in two groups based on result from blood culturing: blood culture negative EOS (n=65) and blood culture positive EOS (n=6).
Sample collection, preparation and analysis
In the previous study, serum had been collected from whole blood collected after insertion of a venous cannula in newborns suspected of EOS and after capillary collection in controls.15 Frozen serum samples had been transported on dry ice to the Netherlands and aliquoted and frozen again on arrival. A stored aliquot was used for the measurement of sP-selectin, sE-selectin, vascular cell adhesion molecule-1 (sVCAM-1), sICAM-1 and sPECAM-1 using the Human Magnetic Bead Adhesion 6-plex panel performance assay (LHC0016M, Thermo Scientific, Waltham, Massachusetts, USA) according to the manufacturer’s instructions. ELISA was used on the same aliquots for measurement of NE (HK319-02, Hycult Biotech, Uden, The Netherlands), MMP-9 (Quantikine DMP900, R&D systems, Minneapolis, Minnesota, USA) and TIMP-1 (Quantikine DTM100, R&D systems), each according to the manufacturers’ instructions. For each molecule, a standard curve was established via which concentrations in neonatal serum were determined. Levels below or above the linear part of this standard curve were reported as the lowest or highest value of the standard curve, respectively. We measured intra-assay variation between plates used in the same assay by calculating coefficient of variation between levels of each molecule in samples from the same patient divided over those plates and accepted a maximum of 20%.
Statistical analysis
A Kruskal-Wallis test with Dunn’s correction for multiple comparisons was used for analysis between the blood parameters and the three groups (blood culture positive EOS, blood culture negative EOS and control groups). <0.05 were considered statistically significant. All analyses were done using Prism V.7.0a (Graphpad Software, San Diego, California, USA).
Results
Demographic variables of the whole study cohort (n=91) can be found in table 1 of Ref. 15. Of baseline characteristics, birth weight, age at presentation (between 0 and 72 hours after birth) and Apgar score at 5 min were distributed unevenly among the three groups (p<0.05). Blood culture results revealed that 6 of 70 newborns with suspected EOS (8.6%; 95% CI 1.9% to 15.3%) had a positive blood culture with gram-negative pathogens Klebsiella pneumoniae (n=2), Enterobacter cloacae (n=2) and Escherichia coli (n=2). One newborn had EOS due to a spontaneous bacterial peritonitis. For n=4 other newborns, cause of EOS was unknown, but they presented with neonatal jaundice (n=1), perinatal asphyxia (n=1), meconium aspiration (n=1) and hypoglycaemia (n=1).
Serum concentrations of soluble endothelial cell adhesion molecules and their sheddases
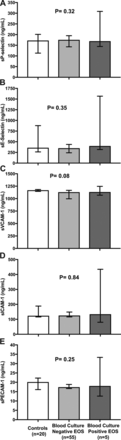
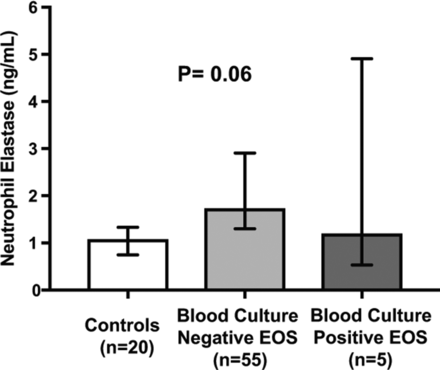
Due to the limited amount of serum available, not all molecules could be measured in all samples. We were able to measure MMP-9 and TIMP-1 levels in serum of n=90 newborns and sCAMs and NE levels in serum of n=80 newborns, respectively. We found no differences in median concentrations of sCAMs (figure 1), MMP-9 and TIMP-1 (figure 2) or NE (figure 3) between blood culture positive EOS, blood culture negative EOS and control groups at start of antibiotic treatment within 72 hours after birth.
Circulating levels of endothelial adhesion molecules sP-selectin, sE-selectin, sVCAM-1, sICAM-1 and sPECAM-1 in Surinamese newborns. (A) sP-selectin; (B) sE-selectin; (C) sVCAM-1; (D) sICAM-1; (E) sPECAM-1. Bars represent median values and error bars 95% CI. P<0.05 were considered statistically significant. EOS, early onset sepsis; sICAM-1, soluble intercellular adhesion molecule-1; sPECAM-1; soluble platelet and endothelial cell adhesion molecule-1; sVCAM-1, soluble vascular cell adhesion molecule-1.
Circulating levels of MMP-9 and TIMP-1, and TIMP-1/MMP-9 ratios in Surinamese newborns. (A) MMP-9; (B) TIMP-1; (C) TIMP-1/MMP-9 ratios. Bars represent median values and error bars 95% CI. P<0.05 were considered statistically significant. EOS, early onset sepsis; MMP-9, matrix metalloproteinase-9; TIMP-1, tissue inhibitor of metalloproteinase.
Circulating levels of neutrophil elastase in Surinamese newborns. Bars represent median values and error bars 95% CI. P<0.05 were considered statistically significant. EOS, early onset sepsis.
Discussion
In this study, we investigated whether sCAMs and their sheddases circulate at higher concentrations in near and at term newborns with blood culture positive EOS at start of antibiotic treatment. In contrast to our hypothesis, none of the molecules showed any difference in serum concentrations between blood culture positive EOS, blood culture negative EOS and controls within 72 hours after birth. Our data indicate that serum concentrations of sCAMs and their sheddases have neither clinical utility as biomarkers for EOS nor to guide the start of antibiotic treatment.
Previously, we found evidence for endothelial cell activation in blood culture positive EOS in the same newborns used for this study, represented by a disbalance in Ang-2/Ang-1 ratio.15 Since the current data demonstrate that this disbalance was not paralleled by increased release of sCAM or sheddases in EOS, we conclude that endothelial CAM shedding is not or to a lesser extent involved in the pathophysiology of EOS. For interpretation of our data, we reviewed and summarised available data on sCAMs and sheddases in newborns with sepsis in online supplementary table 1.16–34 Comparison of our results with other existing data is complicated because of heterogenic makeup of chosen cohorts. Only one study reported a comparable cohort of near and at term newborns with suspected EOS within 72 hours after birth, in whom increased concentrations of sICAM-1 and NE were associated with blood culture positive EOS.19 Other earlier studies compared concentrations of sCAMs in heterogenic cohorts consisting of newborns with different gestational and postnatal ages, either having EOS (based on varying definitions) or sepsis after 72 hours after birth (ie, late onset sepsis). This variation in inclusion criteria is an important confounding factor in the interpretation of the observed concentrations in septic and healthy newborns. Overall, our results are in line with these studies that show that clinical utility of sCAMs and sheddases in EOS is very limited.
Supplementary file 1
In an earlier review by our group, we pooled published data on sCAM concentrations in newborns.7 Soluble CAM concentrations in the current study corresponded well with concentrations discussed in our review and those established in earlier studies in uninfected healthy newborns with similar gestational and postnatal age.7 23 24 29–32 However, MMP-9, TIMP-1 and NE concentrations were different and up to 4-fold, 2-fold and 10-fold higher, respectively, than those reported in earlier studies,17 18 21 22 25 30 which may have been due to other methods used (see limitations). Furthermore, our earlier review and earlier data indicated that significant age-related discrepancies exist in sCAM concentrations between newborns, children and adults. As an example, in at term newborns, sVCAM-1 concentrations in the first postnatal week were almost twice the concentrations in healthy adults and equally high compared with septic adults, suggesting that sVCAM-1 concentrations start of high in early newborn life and then decrease with increasing age.7 31 In our study, concentrations of sCAMs and sheddases during the first 3 days of life in our study remained stable, which was in contrast with earlier work in healthy newborns showing that sE-selectin decreased and sICAM-1 and sVCAM-1 increased between day 1 and 5 after birth, while sPECAM-1 levels did not change.29–32 Even though some discrepancies with earlier reports exist, overall one can conclude that these and our data indicate that concentrations of sCAMs and sheddases measured within 72 hours after birth are high and do not discriminate between septic and healthy newborns, which limits their use as biomarkers for early identification or exclusion of EOS.
Our and pre-existing data suggest that overall high sCAM and sheddase concentrations in newborns are the result of other perinatal factors than EOS. Several pathophysiological processes may explain this premise. Birth may induce a ‘pro-adhesive’ state of the endothelium leading to increased endothelial CAMs expression on, and shedding from, its surface. Additionally, the increase in overall leucocyte numbers and inflammatory activation of subsets associated with human birth, which was shown to be positively associated with increased perinatal stress,35–37 may cause higher intensity of leucocyte-endothelial interactions and subsequent increases endothelial CAMs shedding. Aberrant adhesion of activated leucocytes to activated endothelium is associated with endothelial dysfunction and increased vascular permeability.38 39 Shedding of endothelial CAMs may then result in prevention of aberrant leucocyte adhesion on two complementary levels, namely (1) to lower endothelial CAMs density to prevent adhesion or promote de-adhesion of already adhering leucocytes and (2) to release circulating sCAMs that act as ‘decoy receptors’ to capture leucocytes in the vasculature to limit leucocyte-endothelial interactions.7 10 Whether this occurs in real life and what the contribution is to sCAM and sheddase concentrations in newborns remains unknown and could be studied in neonatal animal models.40–42
Our study has some limitations. First, sample size was relatively small. As a result, logistic regression analysis of other factors, such as maternal perinatal factors or method of birth, potentially influencing levels of sCAMs and sheddases, was precluded. Larger studies in countries such as Suriname, where the incidence of EOS is relatively high in comparison to Western countries,43 are necessary and can contribute to better insight in the vascular pathophysiology of EOS. Second, the use of serum in our study may have caused release of stored pools of MMP-9, TIMP-1 and NE from disrupted leucocytes during the clotting process, which could have accounted for higher levels of these molecules than reported in earlier studies. Last, repeated freeze-thaw cycles may have affected quality of serum samples with regards to reproducibility of NE concentrations.
In conclusion, our data indicate that serum concentrations of sCAMs and sheddases are not higher in Surinamese newborns with EOS versus controls at start of antibiotic treatment. Although concentrations may still increase significantly more in newborns with EOS, other mechanisms, such as perinatal stress during birth, may drive overall high concentrations in all newborns which precludes discrimination between septic and healthy newborns.
Acknowledgments
The research in this study was supported by the Thrasher Research Fund (TRF13064) (R. Zonneveld) and Tergooi Hospitals, Blaricum, The Netherlands. The authors acknowledge the efforts of all employees of the Clinical Laboratory of the Academic Hospital Paramaribo and the Central Laboratory of Suriname, Paramaribo, Suriname, for assistance with sample storage, handling and transport. We would like to thank Dr Ellen Tromp for assistance with the statistical analysis for the final version of this paper.
References
Footnotes
Contributors RZ, MvM, GM and FBP conceived and designed the study. RZ and AJ collected clinical data and collected the samples. RZ, RMJ and MvM prepared the samples and performed the sample analysis. RZ and MvM analysed the final database. RZ, MvM, GM and FBP drafted the manuscript. All authors coauthored and approved the final manuscript.
Funding The Thrasher Research Fund (TRF13064) and Tergooi Hospitals, Blaricum, The Netherlands.
Competing interests None declared.
Patient consent Obtained.
Ethics approval Surinamese Medical Ethical Board.
Provenance and peer review Not commissioned; externally peer reviewed.