Article Text
Abstract
Background Hospital-acquired strains (HASs) and multiresistant strains in neonatal intensive care unit often harbour virulence and resistance mechanisms, carrying the risk of invasive infections. We describe colonisation with Enterobacteriaceae in neonates receiving early directed versus routine family-integrated care (FIC) within the first month of life.
Methods A prospective cohort study included neonates with a gestational age below 34 weeks. During the first period, neonates were admitted to an open bay unit with transfer to the single-family room if available; feeding with the mother’s own breast milk (MOBM) was introduced within 24 hours, and skin-to-skin contact (SSC) within 5 days of life (the routine care group). During the second period, following a wash-in of 2 months, care in a single-family room within 48 hours, the introduction of MOBM within two and SSC in 48 hours were applied (the intervention group). Enterobacteriaceae isolated from neonatal stool, breast milk and parental skin swabs were genotyped, Simpson’s Index of Diversity (SID) calculated, and extended-spectrum beta-lactamases (ESBL) detected.
Results In 64 neonate-parents’ groups, 176 Enterobacteriaceae, 87 in routine care and 89 in the intervention group were isolated; 26 vs 18 were HAS and one vs three ESBL positive, respectively. In the intervention group compared with the routine care group, SSC and MOBM feeding was started significantly earlier (p<0.001); during the first week of life, time spent in SSC was longer (median hours per day 4.8 (4–5.1) vs 1.9 (1.4–2.6), p<0.001) and the proportion of MOBM in enteral feeds was higher (median (IQR) 97.8% (95.1–100) vs 95.1% (87.2–97.4), p=0.011). Compared with the routine care group, the intervention group had higher SID and a reduction of HAS by 33.1% (95% CI 24.4% to 42.4%) in time series analysis.
Conclusions Early implementation of FIC measures may hold the potential to increase diversity and reduce colonisation with HAS Enterobacteriaceae.
- Neonatology
- Microbiology
Data availability statement
No data are available.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
WHAT IS ALREADY KNOWN ON THIS TOPIC
Preterm neonates admitted to neonatal intensive care unit show low diversity of early gut colonisation compared with healthy term infants and are colonised by hospital-acquired strains that are associated with an increased risk of invasive infections.
WHAT THIS STUDY ADDS
Early implementation of family-integrated care measures may increase the diversity and limit colonisation with hospital-acquired strains of Enterobacteriaceae.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This study suggests that early family-integrated neonatal intensive care could reduce levels of hospital-acquired strains and should be assessed in further prospective studies.
Background
Preterm infants are colonised by aerobic or facultative opportunistic pathogens, including Enterobacteriaceae, within the first few weeks of life.1–3 Opportunistic colonisers are usually heterogeneous and dependent on feeding habits, hygiene conditions and antimicrobial treatment.4 These first colonisers may be hospital-acquired strains (HASs) often harbouring antibiotic resistance mechanisms, such as extended-spectrum beta-lactamases (ESBL), and carrying an increased risk of invasive infections,5 especially in preterm infants with the immature immune system, prolonged hospitalisation and frequent use of invasive devices.6 7
Neonatal intensive care units (NICUs) in the developed world are introducing family-integrated care (FIC)8 and change NICU configurations from the traditional open-bay units to single-family rooms9 10 to involve parents in their infant’s care.11 Important principles of FIC12 include rooming-in with family members,13 14 promoting skin-to-skin contact (SSC) and feeding with the mother’s own breast milk (MOBM).15 Recent studies suggest the potential of FIC to reduce the incidence or severity of sepsis16–18 and transmission of multidrug-resistant organisms.19 However, the studies are still scarce and controversial.9 We aimed to compare the early development of gut colonisation with Enterobacteriaceae in preterm neonates receiving routine versus early directed FIC and to identify risk factors associated with the emergence of HAS and ESBL-positive Enterobacteriaceae.
Methods
Study design
We conducted a prospective, two sequential period cohort study in the NICU of East Tallinn Central Hospital (online supplemental figure 1) which was renovated in October 2017.
Supplemental material
During the first period, neonates with gestational age (GA) <34 weeks were admitted either to the open bay unit with late transfer to a single-family room if available. Introduction of feeding with the MOBM within 24 hours and SSC within 5 days of life (routine care group) was accepted as per the routine of the ward. During the second period, following a wash-in period of 2 months, we reinforced early implementation of FIC measures with the goal to admit families into a single-family room before 48 hours, introduce feeding with MOBM within 2 hours and start SSC with both parents within 48 hours of birth. Parental involvement in the baby’s care was encouraged and supported to reduce medical staff contacts during NICU stay (intervention group).
We included inborn neonates and their parents as soon as possible after birth if they (1) needed non-invasive respiratory support; (2) tolerated enteral feeding; (3) the mother was willing to provide MOBM immediately after birth up to the age of 4 weeks and (4) parents agreed to practice SSC. We excluded neonates if they (1) had contraindications to feeding with MOBM (eg, known HIV-positive status); (2) were expected to survive for less than 72 hours or (3) were born from multiple pregnancy. Neonates dropped out from the study if (1) transferred to another hospital within 7 days from birth; (2) the mother or neonate died; (3) MOBM decreased to <10% of the entire volume of daily enteral feeds for more than seven consecutive days; (4) mother consumed probiotics during the study period or (5) for any other reason when the treating physician considered the participation of the child not warranted. Drop-outs were replaced by new recruits.
Patient and public involvement
No patient and public representatives were involved in the design of the study. All eligible patients received detailed information about the trial when they were admitted to the unit and offered a possibility to participate. Study participants, who expressed interest in receiving information on trial results (all participants were asked), will receive an overview of the main results after the final data analysis.
Data collection
The full list of collected demographical and clinical data is presented in online supplemental text. Parents and medical staff filled in a daily diary card as described previously20 registering parental and personnel activities in the care of the infant.
Supplemental material
Sample collection
Neonatal stool, MOBM and the care-taking parents’ skin swabs were collected on admission, at 1 and 4 weeks after delivery. Incubator linen and nasal Continuous Positive Airway Pressure (nCPAP) prongs (hereafter referred to as environment) were sampled once within the first 48 hours of life. NICU doors and the MOBM refrigerator handle, personnel hands, computer keyboard and alarm knob in the stabilisation room (hereafter referred to as unit-related environment) were sampled at the start and end of each period.
Stool samples were collected with a sterile spatula into a sterile container. A skin area of about 10 cm2 of the décolletage area was rubbed with a swab moistened in normal saline and placed into Amies transport medium without charcoal (Copan Italia spa, Brescia, Italy). Environmental samples were handled similarly. MOBM samples were collected as described previously.21 Stool and MOBM samples were stored at −20°C until transported to −80°C within 96 hours; skin and environmental swabs were stored at +4°C until cultured within 72 hours.
Samples were plated onto MacConkey agar, incubated at 37°C for 18–24 hours. Two to three colonies with distinct morphology were randomly selected and identified to the species level by matrix-assisted laser desorption/ionisation time-of-flight mass-spectrometry (Bruker Daltonics, Bremen, Germany). Of morphologically distinct types, only one isolate of each species for all time points and materials was randomly chosen for further analysis. The presence of ESBL was detected by Chromatic ESBL media (Liofilchem, Italy) and cefpodoxime disks (10 µg).22 All Enterobacteriaceae were typed by pulsed-field gel electrophoresis (PFGE) with each distinct PFGE type marked by a randomly assigned number.23 HAS was defined according to a similar PFGE pattern seen in more than one neonate/parents/environment group (further defined as a family group).
Statistical analysis
This was a substudy of a larger study focusing on early colonisation with coagulase-negative staphylococci. With a two-sided alpha level of 5% and power of 80%, 32 neonates per group were required to detect an increase in the proportion of preterm neonates colonised with Staphylococcus epidermidis strains from MOBM from 14% (observed in our previous study) to 42%.24 The software programs Sigma Plot for Windows V.11.0 (GmbH Formation, Germany); and V.R 2.6.2 (A Language and Environment, http://www.r-project.org) were used. The routine care group and the intervention group were compared by t-test or Mann-Whitney U test (continuous variables) and χ2 or Fisher’s exact test (categorical variables).
Univariate logistic regression models were applied to determine risk factors for cumulative colonisation of any studied site (including the neonatal gut, MOBM and parents’ skin) with (1) hospital-acquired and (2) ESBL strains of Enterobacteriaceae by week 4 within a family group. The following independent variables were included: study group, GA, mode of delivery, presence of premature rupture of membranes, maternal antibiotics, time of first MOBM and proportion of MOBM in enteral feeds, time of first and total duration of SSC during NICU stay, duration of hospitalisation, use and duration of central venous and umbilical arterial catheter and unit personnel contact time with the newborn.
All variables that were significant (at a p<0.1) in the univariate logistic regression model were included into the multivariable logistic regression model with subsequent removal in order of insignificance until only variables with p<0.05 were retained. To avoid collinearity, only one of the highly correlated variables was included at a time.
Simpson’s Index of Diversity (SID) based on all PFGE types in study groups, with 95% CIs, was calculated using the Comparing Partitions website (http://www.comparingpartitions.info/?link=Tool). The proportion of HAS among all isolates was analysed as time series data using autoregressive integrated moving average (ARIMA) with the study group as an exogenous variable. Arcsine square root transformation was applied to the proportion prior to the model fitting. Stationarity of the time series was tested with Dickey-Fuller test and potential ARIMA orders determined using autocorrelation and partial autocorrelation plots. Subsequently, Akaike information criterion was used to determine the best model. ARIMA model was accepted if residuals were white noise and Ljung-Box test indicated no autocorrelation.
Results
Study population
During the two study periods 74, eligible family groups were identified of whom 64 neonates (n=32 in each group) with 110 parents (46 fathers) completed the study. Reasons for exclusion and drop-out are shown in online supplemental figure 1 and online supplemental table 1. In the intervention group compared with the routine group, SSC and MOBM feeding was started significantly earlier; during the first week of life, time spent in SSC was longer (median h per day 4.8 (4–5.1) vs 1.9 (1.4–2.6), p<0.001) and the proportion of MOBM in enteral feeds was higher (median (IQR) 97.8% (95.1–100) vs 95.1% (87.2–97.4), p=0.011) (table 1). NICU personnel spent significantly more time in contact with neonates in the routine care compared with the intervention group (median (IQR) 33 (26–43) vs 22 (12–27) minutes per day; p<0.001). None of the infants died or had necrotising enterocolitis.
Supplemental material
Demographic and clinical characteristics
Similar proportions of neonates in routine care and the intervention group became colonised by Enterobacteriaceae by the end of the first (69% and 50%) and fourth week of life (97% and 91%), respectively (online supplemental file 2). The colonisation of MOBM as well as parental skin by Enterobacteriaceae was also similar and remained low (<35%) throughout both study periods (online supplemental figure 2B). A total of 176 (neonatal gut n=134, MOBM n=19, parents’ skin n=16, nCPAP n=2 and incubator linen n=5) Enterobacteriaceae isolates were detected, 87 in the routine care and 89 in the intervention group (table 2). The most frequent micro-organisms in the routine care group were Escherichia coli and Enterobacter cloacae, while in the intervention group Klebsiella oxytoca and Enterobacter cloacae predominated (table 2; online supplemental figure 1A). The proportion of E. coli was higher (27.6% vs 14.6%; p=0.018) and K. oxytoca lower (14.9% vs 34.8%; p=0.007) in routine care compared with the intervention group, respectively (figure 1A). Colonisation of nCPAP equipment and incubator linen (three E. cloacae, three E. coli and one Citrobacter amalonaticus isolate) was observed only in the intervention group (table 2).
Supplemental material
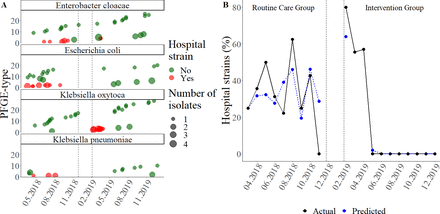
Colonisation with Enterobacteriaceae, HAS and ESBL positive strains in two study groups
Overtime spread by family unit (A) and measured and modeled overall proportion (B) of hospital-acquired strains of Enterobacteriaceae during the two study groups. In (A), the y-axis shows PFGE-type and x-axis shows study the period (time from the birth of the first newborn included in the study till the last newborn concluded the study). Family units colonised by HAS of the specified Enterobacteriaceae species are marked by red and all others colonised by at least one nonhospital strain of the specified Enterobacteriaceae species by green dots. Vertical dotted lines between two periods indicate the interim period. The size of the node is proportional to the number of isolates within the family group. In (B), the actual proportion of hospital strains among all Enterobacteriaceae isolates by month is shown in black dots and the line. The blue line and dots represent the proportion of hospital-acquired strains as predicted by the autoregressive integrated moving average model. The dotted lines between the two periods represent the interim period. Note that the discontinuities of the lines are due to the non-availability of the data for the proportion of the hospital strains and study intervention during the interim period. HAS, hospital-acquired strain; PFGE, pulsed-field gel electrophoresis.
The 176 isolates of Enterobacteriaceae represented 113 distinct PFGE types, 55 in the intervention and 58 in the routine care group. The diversity was lower in routine care compared with the intervention group (SID (95% CI) 0.847 (0.792 to 0.901) vs 0.938 (0.916 to 0.959), respectively; p=0.003). Within family groups, in 8/32 (25%) of routine care and 7/32 (21.9%) of the intervention group similar PFGE types were found in more than one location, predominantly in gut and MOBM (7/8; 87.5%) in routine care and in environment and gut (4/7; 57.1%) in the intervention group (online supplemental figure 3). In the routine care group, neonatal gut colonisation preceded MOBM (4/7) while nCPAP nasal prongs and/or bedsheet colonisation prior to isolation of the same strain from the gut was seen in the intervention group (4/4). There were no isolates of Enterobacteriaceae from any unit-related environment samples.
Supplemental material
Colonisation with HAS and ESBL-positive strains
HAS constituted 25% (44/176) of all Enterobacteriaceae isolates with similar proportions observed in routine care and the intervention group (26/87; 29.9% and 18/89; 20.2%). They represented 10 different PFGE types; 7 in routine care and 3 in the intervention group. The dominant HAS in the routine care group were E. coli (12/26 isolates, representing three PFGE types) and E. cloacae (7/26, representing 3 PFGE types), followed by K. pneumoniae and Enterobacter asburiae (5/26 and 2/26, both representing 1 PFGE type). In the intervention group, K. oxytoca (16/18, representing two PFGE types) dominated, followed by E. cloacae (2/18, representing one PFGE type). HASs were isolated from the gut at least once in 16/32 and 8/32 neonates in routine care and the intervention group, respectively (p=0.07) (online supplemental figure 2B). MOBM and parental skin colonisation with Enterobacteriaceae HAS was rare (<16%) in both study periods (table 2, online supplemental figure 2B).
While the spread of HAS was seen throughout the routine care period, in the intervention group they were isolated only at the beginning (figure 1A). Time series analysis (ARIMA model) showed that study intervention reduced the proportion of HAS among isolates by 33.1% (95% CI 24.4% to 42.4%) with a time delay of 3 months (figure 1B).
One (3.1%) and 3 (9.4%; in 1 of these neonates 2 different species) neonates became colonised with a total of two and seven ESBL-positive strains in the routine care and the intervention group, respectively. ESBL-positive strains were distributed between the routine care/intervention group and samples as follows: in gut 1/4 (E. cloacae / E. coli, E. cloacae, 2 K. oxytoca); in MOBM 1/1 (K. oxytoca / E. cloacae), on skin 0/1 (K. oxytoca) and on nCPAP 0/1 (E. coli). All ESBL-positive strains represented different PFGE types.
Factors influencing colonisation with HAS and ESBL-positive strains
According to univariate logistic regression, in the intervention group, higher birth weight (BW) and GA were associated with lower odds of colonisation with HAS of any site within the family group (table 3). In multivariable logistic regression analysis, no statistically significant associations with the odds of colonisation with HAS were found. In univariate analysis, the higher odds of colonisation with ESBL-positive strains were associated with higher BW and a larger volume of total enteral feed during the NICU stay and lower odds with a higher volume of MOBM during the first week of life (table 3). Due to a small number of colonisation events, multivariable logistic regression was not performed.
Risk factors of cumulative colonisation with hospital and ESBL strains by week 4 (results of univariate regression analysis) at any site/material in family group
Discussion
Our study showed that early directed implementation of FIC measures in NICU has the potential to increase the diversity of Enterobacteriaceae and reduce gut colonisation by HAS, known to be more virulent than community acquired strains. In addition, we showed that lower BW and GA promote colonisation by HAS.
Multiple components of our intervention likely contributed to the reduced spread of HAS, differences in species structure, and higher diversity as reflected by SID, in the intervention group. First, it included early admission to a single-family room. Although single-family rooms were partly used during the routine care period as well, in the intervention group all neonates were transferred significantly earlier. A few studies have shown that single-family room design in NICU19 and in adult ICU25 can substantially reduce the acquisition of multidrug resistant or pathogenic microorganisms. In contrast, another study found relatively more differentially abundant antimicrobial resistance genes in all sample types (stool, skin and environment) in a ‘new single-family room NICU’ compared with an ‘old open bay unit’ with no difference in species alpha diversity.10
Second, there is little doubt about the role of the timing, volume and type of enteral feeds in early neonatal gut colonisation, although the exact effects are more difficult to define.26 In our study, the intervention involving earlier and higher volume MOBM feeding as one component was associated with higher diversity of Enterobacteriaceae. The high prevalence of K. oxytoca in the intervention group is consistent with previous studies showing that enteral feeding, especially with MOBM, increases gut colonisation with Gram-negative opportunistic bacteria, including high amounts of K. oxytoca.23 27 However, in our study, the majority of K. oxytoca isolates in the intervention group were HAS representing just two PFGE types and in only one case the same PFGE type was isolated from MOBM prior to neonatal gut colonisation. Similarly, a large proportion of the predominant E. coli strains in the routine care group (12/26) were HAS representing just 3 PFGE types. The NICU flora is diverse and closely related to the infants’ microbiome.28 Findings by Brooks et al29 point to a scenario in which gut microbes are introduced from room sources, thrive in the gut, and are further disseminated to the immediate environment, creating a cycle of room to infant/parent colonisation. In our study, in many cases a micro-organism appeared in MOBM only after primary neonatal gut colonisation, suggesting this route of transmission. Our previous study of early coagulase-negative staphylococcal colonisation in NICU suggested a similar course of events.23 According to time series analysis, the proportion of HAS in an intervention group dropped, although after a lag period. Thus, the third reason for the decrease in HAS could be a reduced transmission from neonates to personnel/environment and vice versa because of fewer contacts, leading to a reduction of reservoirs within the unit although after a delay, possibly due to the gradual manifestation of the effect of the intervention.
The association between lower BW and GA and higher odds of colonisation with HAS strains in our study once again corroborates the well-recognised role of patient characteristics in early gut colonisation in NICU. An immature immune system30 and the need for more medical interventions31 with frequent direct and indirect contacts with healthcare workers predispose them to various transmission routes. However, due to similar demographic characteristics of the neonates in both study groups such an association unlikely contributed to the reduced occurrence of HAS in the intervention group.
The study has several limitations. First, we followed colonisation over a relatively short period and the count of Enterobacteriaceae species and hospital strains was small. However, given that the incidence of late-onset sepsis in preterm infants peaks by the 7th to 22nd day of life26 and the possible role of early colonisers, interventions targeting primarily the first week of life, hold potential for prevention. Second, the relatively small study groups together with the low prevalence of ESBL strains did not allow for drawing firm conclusions on possible transfer routes and risk factors of colonisation with these resistant bacteria. Third, being aware of the potential benefits of randomisation, we conducted a cohort study for ethical and practical reasons. We believe, that conducting a randomised trial would have carried a high risk of contamination of the routine care group, as the intervention involves a behavioural change in the unit. Based on the number and reasons for exclusion and drop-out from the study as well as the similar GA distribution and severity of the clinical condition of study participants during the two periods, no major bias can be suspected. Fourth, the relatively short interim period of 2 months between the two study periods may have been insufficient to fully change the everyday work culture. Time lag in the effect of the FIC bundle on colonisation with HAS supports this assumption. Transition to the renovated new single-family room unit 6 months prior to the start of the study may also have contributed into the change in the NICU microbiota.
Conclusions
Early implementation of FIC measures may hold the potential to increase microbial diversity and reduce colonisation with HAS of Enterobacteriaceae. Lower GA and BW were associated with a higher risk of HAS transmission. Colonisation with ESBL strains appears to be related to feeding strategy, with lower risk associated with the lower total volume of enteral feed and higher proportion of MOBM in enteral feeds. Early gut colonisation of neonates in NICU is a complex process that offers the potential to improve short-term and long-term health outcomes for this vulnerable group. Further studies to improve our understanding of the interplay within this multimodal process are urgently needed.
Data availability statement
No data are available.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants and was approved by Research Ethics Committee of the University of Tartu (Approval no 276/T-10). Participants gave informed consent to participate in the study before taking part.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
ÜP and AT-V contributed equally.
Contributors ÜP: conceptualisation, methodology, software, validation, formal analysis, investigation, resources, data curation, term, writing—original draft, editing, visualisation; AT-V: conceptualisation, software, validation, formal analysis, resources, data curation, term, writing—original draft, editing, visualisation; HS: software, validation, formal analysis, writing—review and editing, visualisation, supervision; JŠ: resources; KT: resources; SV: software; IL: conceptualisation, writing—review and editing, supervision, project administration, funding acquisition; TM: the guarantor, conceptualisation, term, writing—review and editing, supervision, project administration.
Funding This study was supported by a grant from the Estonian Research Council IUT 34–24 and supported by the Estonian Ministry of Education and Research (Grant No. KOGU- 401 HUMB).
Competing interests No, there are no competing interests.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.