Abstract
Purposes
Tacrolimus (TAC) is the most widely used immunosuppressant for the prevention of acute rejection after solid organ transplantation. Its pharmacokinetics (PK) show considerable variability, making TAC a good candidate for therapeutic drug monitoring (TDM). The principal aim of the study was to describe the PK of TAC in pediatric patients during the first year after transplantation.
Methods
Routine TDM trough levels of TAC were obtained from 42 pediatric liver allograft recipients during the first year after transplantation. A population PK model was developed using nonlinear mixed-effects modeling to describe TAC PK during this period and to explain the observed variability by means of patients’ demographics, biochemical test results and physiological characteristics.
Results
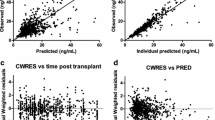
The PK of TAC were best described by a two-compartment model with first-order elimination. Apparent volumes of the central compartment, intercomparmental clearance and maximum blood clearance estimates were 253 L, 115 L/day and 314 L/day, respectively. The absorption first-order rate and volume of peripheral compartment were fixed to 4.5 h-1 and 100 L, respectively. While hematocrit levels, time after transplantation and bodyweight influenced TAC clearance, bodyweight was the only covariate retained on volume of distribution.
Conclusions
We developed a TAC population PK model in pediatrics covering the first year after liver transplantation that may serve as a tool for TAC dose individualization as part of TDM.




Similar content being viewed by others
References
Flanagan WM, Corthésy B, Bram RJ, Crabtree GR (1991) Nuclear association of a T-cell transcription factor blocked by FK-506 and cyclosporin A. Nature 352(6338):803–807
Kelly D (2006) Current issues in pediatric transplantation. Pediatr Transplant 10(6):712–720
Staatz CE, Tett SE (2004) Clinical pharmacokinetics and pharmacodynamics of tacrolimus in solid organ transplantation. Clin Pharmacokinet 43(10):623–653
Naesens M, Kuypers D, Sarwal M (2009) Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol 4(2):481–508
Rodriguez-Peralverez M, Germani G, Darius T, Lerut J, Tsochatzis E, Burroughs AK (2012) Tacrolimus trough levels, rejection and renal impairment in liver transplantation: a systematic review and meta-analysis. Am J Transplant 12:2797–2814
D’Antiga L, Dhawan A, Portmann B, Francavilla R, Rela M, Heaton N, Mieli-Vergani G (2002) Late cellular rejection in paediatric liver transplantation: aetiology and outcome. Transplantation 73(1):80–84
Staatz CE, Goodman LK, Tett SE (2010) Effect of CYP3A and ABCB1 single nucleotide polymorphisms on the pharmacokinetics and pharmacodynamics of calcineurin inhibitors: part I. Clin Pharmacokinet 49(3):141–175
Willis C, Staatz CE, Tett SE (2003) Bayesian forecasting and prediction of tacrolimus concentrations in pediatric liver and adult renal transplant recipients. Ther Drug Monit 25(2):158–166
Yasuhara M, Hashida T, Toraguchi M et al (1995) Pharmacokinetics and pharmacodynamics of FK 506 in pediatric patients receiving living-related donor liver transplantations. Transplant Proc 27(1):1108–1110
Sam WJ, Aw M, Quak SH, Lim SM, Charles BG, Chan SY, Ho PC (2000) Population pharmacokinetics of tacrolimus in Asian pediatric liver transplant patients. Br J Clin Pharmacol 50(6):531–541
Sam WJ, Tham LS, Holmes MJ, Aw M, Quak SH, Lee KH, Lim S et al (2006) Population pharmacokinetics of tacrolimus in whole blood and plasma in asian liver transplant patients. Clin Pharmacokinet 45(1):59–75
Staatz CE, Taylor PJ, Lynch SV, Willis C, Charles BG, Tett SE (2001) Population pharmacokinetics of tacrolimus in children who receive cut-down or full liver transplants. Transplantation 72(6):1056–1061
García Sánchez MJ, Manzanares C, Santos-Buelga D, Blázquez A, Manzanares J, Urruzuno P, Medina E (2001) Covariate effects on the apparent clearance of tacrolimus in pediatric liver transplant patients undergoing conversion therapy. Clin Pharmacokinet 40(1):63–71
Fukudo M, Yano I, Masuda S et al (2006) Population pharmacokinetic and pharmacogenomic analysis of tacrolimus in pediatric living-donor liver transplant recipients. Clin Pharmacol Ther 80(4):331–345
Wallin JE, Bergstrand M, Wilczek HE, Nydert PS, Karlsson MO, Staatz CE (2011) Population pharmacokinetics of tacrolimus in pediatric liver transplantation: early posttransplantation clearance. Ther Drug Monit 33(6):663–672
de Wildt SN, van Schaik RH, Soldin OP et al (2011) The interactions of age, genetics, and disease severity on tacrolimus dosing requirements after pediatric kidney and liver transplantation. Eur J Clin Pharmacol 67(12):1231–1241
Antignac M, Hulot JS, Boleslawski E, Hannoun L, Touitou Y, Farinotti R, Lechat P, Urien S (2005) Population pharmacokinetics of tacrolimus in full liver transplant patients: modeling of the post-operative clearance. Eur J Clin Pharmacol 61(5–6):409–416
Staatz CE, Willis C, Taylor PJ, Lynch SV, Tett SE (2003) Toward better outcomes with tacrolimus therapy: population pharmacokinetics and individualized dosage prediction in adult liver transplantation. Liver Transpl 9(2):130–137
Fukatsu S, Yano I, Igarashi T et al (2001) Population pharmacokinetics of tacrolimus in adult recipients receiving living-donor liver transplantation. Eur J Clin Pharmacol 57(6–7):479–484
Zahir H, McLachlan AJ, Nelson A, McCaughan G, Gleeson M, Akhlaghi F (2005) Population pharmacokinetic estimation of tacrolimus apparent clearance in adult liver transplant recipients. Ther Drug Monit 27(4):422–430
Blanchet B, Duvoux C, Costentin CE et al (2008) Pharmacokinetic-pharmacodynamic assessment of tacrolimus in liver-transplant recipients during the early post-transplantation period. Ther Drug Monit 30(4):412–418
Li D, Lu W, Zhu JY, Gao J, Lou YQ, Zhang GL (2007) Population pharmacokinetics of tacrolimus and CYP3A5, MDR1 and IL-10 polymorphisms in adult liver transplant patients. J Clin Pharm Ther 32(5):505–515
Oteo I, Lukas JC, Leal N, Suarez E, Valdivieso A, Gastaca M, Ortiz de Urbina J, Calvo R (2013) Tacrolimus pharmacokinetics in the early post-liver transplantation period and clinical applicability via Bayesian prediction. Eur J Clin Pharmacol 69:65–74
Wallemacq P, Armstrong VW, Brunet M et al (2009) Opportunities to optimize tacrolimus therapy in solid organ transplantation: report of the European consensus conference. Ther Drug Monit 31(2):139–152
Sheiner LB, Beal SL (1983) Evaluation of methods for estimating population pharmacokinetic parameters. III. Monoexponential model: routine clinical pharmacokinetic data. J Pharmacokinet Biopharm 11(3):303–319
Alak AM, Cook M, Bekersky I (1997) Measurement of tacrolimus (FK506) and its metabolites: a review of assay development and application in therapeutic drug monitoring and pharmacokinetic studies. Ther Drug Monit 19(1):338–351
Wallemacq P, Goffinet JS, O’Morchoe S et al (2009) Multisite analitical evaluation of the Abbott ARCHITECT tacrolimus assay. Ther Drug Monit 31(2):198–204
Lindbom L, Pihlgren P, Jonsson EN (2005) PsN-Toolkit—a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput Methods Programs Biomed 79:241–257
Bertrand J, Comets E, Mentre F (2008) Comparison of model-based tests and selection strategies to detect genetic polymorphisms influencing pharmacokinetic parameters. J Biopharm Stat 18(6):1084–1102
Hooker AC, Staatz CE, Karlsson MO (2007) Conditional weighted residuals (CWRES): a model diagnostic for the FOCE method. Pharm Res 24(12):2187–2197
Schwartz GJ, Work DF (2009) New equations to estimate GFR in children with CKD. Clin J Am Soc Nephrol 4(11):1832–1843
Comets E, Brendel K, Mentré F (2008) Computing normalised prediction distribution errors to evaluate nonlinear mixed-effect models: the NDPE add-on package for R. Comput Methods Programs Biomed 90(2):154–166
Jusko WJ, Piekoszewski W, Klintmalm GB et al (1995) Pharmacokinetics of tacrolimus in liver transplant patients. Clin Pharmacol Ther 57(3):281–290
Zhao W, Elie V, Roussey G et al (2009) Population pharmacokinetics and pharmacogenetics of tacrolimus in de novo pediatric kidney transplant recipients. Clin Pharmacol Ther 86(6):609–618
Zhao W, Fakhoury M, Baudouin V et al (2013) Population pharmacokinetics and pharmacogenetics of once daily prolonged-release formulation of tacrolimus in pediatric and adolescent kidney transplant recipients. Eur J Clin Pharmacol 69(2):189–195
Uesugi M, Masuda S, Katsura T et al (2006) Effect of intestinal CYP3A5 on postoperative tacrolimus trough levels in living-donor liver transplant recipients. Pharmacogenet Genomics 16(2):119–127
Acknowledgments
We thank the pediatric coordinator Magda Janssens for patients’ data research support.
Conflict of interest statement
The authors declare no conflict of interest related to this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guy-Viterbo, V., Scohy, A., Verbeeck, R.K. et al. Population pharmacokinetic analysis of tacrolimus in the first year after pediatric liver transplantation. Eur J Clin Pharmacol 69, 1533–1542 (2013). https://doi.org/10.1007/s00228-013-1501-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-013-1501-0