Abstract
Introduction
Neurodevelopmental outcome in prematures who suffer from a neonatal brain injury depends on the lesion itself, and on how the lesion interferes with the still developing functional anatomy.
Methods
Most of the neuronal migration is completed by midgestation. The second part of the gestation corresponds to the development of the connectivity and sulcation, of the maturation of the oligodendrocytic lineage and of the microglia, and of the vascular bed in the parenchyma beyond the germinal matrix.
Results
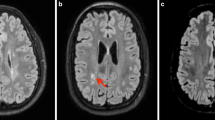
In this paper, the main processes of the developmental anatomy of the premature brain are reviewed, and are correlated with the findings in a prospective series of 105 premature infants born between 24 and 32 weeks of gestation, and serially examined with MR imaging at birth, at term-equivalent age, at 2 years, and at 4 years. Special emphasis was placed (1) on the intraventricular hemorrhage because of the resulting destruction of the germinal matrix and its impact on the late cellular production, (2) on the periventricular venous hemorrhagic infarction because of the selective destruction of the intermediate zone which is associated, and (3) on the apparently perivenous punctate lesions of the white matter because they involve the intermediate zone also, because they have no convincing explanation yet, and because the microglia seems to be associated with their pathogenesis.
Conclusion
These deep venous injuries appear to preserve the subplate zone, which is likely to be a significant element to consider in the perspective of the neurodevelopmental outcome.












Similar content being viewed by others
Abbreviations
- CP:
-
Cortical plate
- CST:
-
Cortico-spinal tract
- DTI:
-
Diffusion tensor imaging
- DWI/ADC:
-
Diffusion-weighted imaging–average diffusion coefficient
- GA:
-
Gestational age
- GE:
-
Ganglionic eminence
- GM:
-
Germinal matrix
- HIE:
-
Hypoxic-ischemic encephalopathy
- IVH:
-
Intra-ventricular hemorrhage
- IZ:
-
Intermediate zone
- MRS:
-
Magnetic resonance spectroscopy
- MTR:
-
Magnetization transfer ration
- MZ:
-
Marginal zone
- NICU:
-
Neonatal intensive care unit
- PLIC:
-
Posterior limb of the internal capsule
- PP:
-
Pre-plate
- PVHI:
-
Periventricular venous hemorrhagic infarction
- PVL:
-
Peri-ventricular leukomalacia
- PVZ:
-
Peri-ventricular zone
- SP:
-
Sub-plate
- SVZ:
-
Subventricular zone
- TEA:
-
Term-equivalent age
- VZ:
-
Ventricular zone
References
Volpe JJ (2009) Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol 8(1):110–124
Nossin-Manor R, Chung AD, Morris D et al (2011) Optimized T1- and T2-weighted volumetric brain imaging as a diagnostic tool in very preterm neonates. Pediatr Radiol 41:702–710
Counsell SJ, Allsopp JM, Harrison MC et al (2003) Diffusion weighted imaging of the brain in preterm infants with focal and diffuse white matter abnormality. Pediatrics 112:1–7
Inder TE, Warfield SK, Wang H et al (2005) Abnormal cerebral structure is present at term in premature infants. Pediatrics 115:286–294
Leijser LM, de Bruïne FT, Steggerda SJ et al (2009) Brain imaging findings in very preterm infants throughout the neonatal period: Part I. Incidence and evolution of lesions, comparison between ultrasound and MRI. Early Human Devel 85:101–109
Hagman CF, De Vita E, Brainbridge A et al (2009) T2 at MR imaging is an objective quantitative measure of cerebral white matter signal intensity abnormality in preterm infants at term-equivalent age. Radiology 252:209–217
Brown NG, Inder TE, Bear MJ et al (2009) Neurobehavior at term and white and gray matter abnormalities in very preterm infants. J Pediatr 155:32–38
Jinnou H, Kouwaki M, Kiyosawa S, Yokochi K (2009) Neonatal MRI in preterm infants with periventricular leukomalacia and mild disability. Pediatr Internat 51:780–785
Hart AR, Smith MF, Rigby AS et al (2010) Appearances of diffuse excessive high signal intensity (DEHSI) on MR imaging following preterm birth. Pediatr Radiol 40:1390–1396
Boardman JP, Craven C, Valappil S et al (2010) A common neonatal image phenotype predicts adverse neurodevelopmental outcome in children born preterm. Neur Image 52:409–414
de Bruïne FT, van den Berg-Huysmans AA, Leijser LM et al (2011) Clinical implications of MR imaging findings in the white matter in very preterm infants. Radiology 261:899–906
Jeon TY, Kim JH, Joo SY et al (2012) Neurodevelopmental outcomes in preterm infants: comparison of infants with and without diffuse excessive high signal intensity on MR images at near-term-equivalent age. Radiology 263:518–526
Smyser CD, Kidokoro H, Inder TE (2012) Magnetic resonance imaging of the brain at term equivalent age in extremely premature neonates: to scan or not to scan? Child Health 48:794–800
Counsell SJ, Rutherford MA, Cowan FM, Edwards AD (2003) Magnetic resonance imaging of preterm brain injury. Arch Dis Child Fetal Neonatal Ed 88:F269–F274
Miller SP, Ferreiro DM, Leonard C et al (2005) Early brain injury in premature newborns detected with magnetic resonance imaging is associated with adverse early neurodevelopmental outcome. J Pediatr 147:609–616
Dyet LE, Kennea N, Counsell SJ et al (2006) Natural history of brain lesions in extremely preterm infants studied with serial magnetic resonance imaging from birth and neurodevelopmental assessment. Pediatrics 118:536–548
Glass HC, Bonifacio SL, Sullivan J et al (2009) Magnetic resonance imaging and ultrasound injury in preterm infants with seizures. J Child Neurol 24:1105–1111
Rutherford MA, Supramaniam V, Ederies A et al (2010) Magnetic resonance imaging of white matter disease of prematurity. Neuroradiology 52:505–521
Niwa T, de Vries LS, Benders MJNL et al (2011) Punctate white matter lesions in infants: new insights using susceptibility-weighted imaging. Neuroradiology 53:669–679
Bystron I, Blakemore C, Rakic P (2008) Development of the human cerebral cortex: Boulder Committee revisited. Nature Rev Neurosci 9:110–122
Kanold PO, Luhmann HJ (2010) The subplate and early cortical circuits. Ann Rev Neurosci 33:23–48
Kostovic I, Judas M, Rados M, Hrabac P (2002) Laminar organization of the human fetal cerebrum revealed by histochemical markers and magnetic resonance imaging. Cerebral Cortex Mon 12:536–544
Montiel JF, Wang WZ, Oeschger FM et al (2011) Hypothesis on the dual origin of the mammalian subplate. Front Neuroanatomy 1:5–25
Judas M, Sedmak G, Pletikos M, Jovanov-Milosevic N (2010) Populations of subplate and interstitial neurons in fetal and adult human telencephalon. J Anat 217:381–399
Kriegstein AR, Noctor SC (2004) Pattern of neuronal migration in the embryonic cortex. Trends Neurosci 27:392–399
Faux C, Rakic S, Andrews W, Britto JM (2012) Neurons on the move: migrations and lamination of cortical interneurons. Neurosignals 20:164–185
Del Bigio MR (2011) Cell proliferation in human ganglionic eminence and suppression after prematurity-associated haemorrhage. Brain 134:1344–1361
Haynes RL, Borenstein NS, Desilva TM, Folkerth RD et al (2005) Axonal development in the cerebral white matter of the human fetus and infant. J Comp Neurol 484:156–167
ten-Donkelaar HJ, Lammens M, Hori A (2006) Clinical neuroembryology. Springer, Berlin
Marin-Padilla M (1970) Prenatal and early postnatal ontogenesis of the human motor cortex: a Golgi study. I. The sequential development of the cortical layers. Brain Res 23:167–183
Smart IHM, McSherry GM (1986) Gyrus formation in the cerebral cortex of the ferret. II. Description of the internal histological changes. J Anat 147:27–43
Ferrer I, Hernandez-Marti M, Bernet E, Galofre E (1988) Formation and growth of the cerebral convolutions. I. Postnatal development of the median-suprasylvian gyrus and adjoining sulci in the cat. J Anat 160:89–100
Ferrer I, Hernandez-Marti M, Bernet E, Calopa M (1989) Formation and growth of the cerebral convolutions. II. Cell death in the gyrus suprasylvius and adjoining sulci in the cat. Develop Brain Res 45:303–308
Sarnat HB, Flores-Sarnat L (2013) Radial microcolumnar cortical architecture: maturational arrest or cortical dysplasia. Pediatr Neurol 48:259–270
Chi JG, Dooling EC, Gilles FH (1977) Gyral development of the human brain. Ann Neurol 1:86–93
du Plessis A (2009) Cerebral blood flow and metabolism in the developing fetus. Clin Perinatol 36:531–548
Streeter GL (1918) The developmental alterations in the vascular system of the brain of the human embryo. Contrib Embryol 271(24):5–38
Klosovskii BN (1963) Chapter 1. Fundamental facts concerning the stages and principles of development of the brain and its response to noxious agents. In: The development of the brain and its disturbance by harmful factors. Pergamon Press, London, pp 3–43
Sabin FR (1917) Preliminary note on the differentiation of angioblasts and the method by which they produce blood-vessels, blood-plasma and red blood-cells as seen in the living chick. Anat Rec 13:199–204
Sabin FR (1917) Origin and development of the primitive vessels of the chick and the pig. Contrib Embryol 226(6):61–124
Allsopp G, Gamble HJ (1979) Light and electron microscopic observations on the development of the blood vascular system of the human brain. J Anat 128:461–477
Kuban KC, Gilles FH (1985) Human telencephalic angiogenesis. Ann Neurol 17:539–548
Norman MG, O’Kusky JR (1986) The growth and development of the microvasculature in the human cortex. J Neuropathol Exp Neurol 45:222–232
Padget DH (1957) The development of the cranial venous system in man from the view point of comparative anatomy. Contrib Embryol 247:81–140
Raybaud C (2010) Normal and abnormal embryology and development of the intracranial vascular system. Neurosurg Clin N Am 21:399–426
Marin-Padilla M (2012) The human brain microvascular system: development and structure. Front Neuroanat 6:38
Mito T, Konomi H, Houdou S, Takashima S (1991) Immunohistological study of the vasculature of the developing brain. Pediatr Neurol 7:18–22
Ballabh P, Braun A, Nedergaard M (2004) Anatomic analysis of blood vessels in germinal matrix, cerebral cortex, and white matter in developing infants. Pediatr Res 56:117–124
Anstrom JA, Thore CR, Moody DM, Brown WR (2007) Immunolocalization of tight junction proteins in blood vessels in human germinal matrix and cortex. Histochem Cell Biol 127:205–213
Anstrom JA, Brown WR, Moody DM et al (2004) Subependymal veins in premature neonates: implications for hemorrhage. Pediatr Neurol 30:46–53
Nakamura Y, Okudera T, Hashimoto T (1994) Vascular archtecture in white matter of neonates: its relationship to periventricular leukomalacia. J Neuropathol Exp Neurol 53:582–589
Okudera T, Huang PY, Fukusumi A et al (1999) Micro-angiographical studies of the medullary venous system of the cerebral hemisphere. Neuropathol 19:93–111
Degani S (2009) Evaluation of fetal cerebrovascular circulation and brain development: the role of ultrasound and Doppler. Semin Perinatol 33:259–269
Cruz-Martinez R, Figueras F, Hernandez-Andrade E et al (2011) Normal reference ranges of fetal regional blood perfusion as measured by fractional moving blood volume. Ultrasound Obstet Gynecol 37:196–201
Altman DI, Powers WJ, Perlman JM et al (1988) Cerebral blood flow requirement for brain viability in newborn infants is lower than in adults. Ann Neurol 24:218–226
Børch K, Greisen G (1998) Blood flow distribution in the human preterm brain. Pediatr Res 43:28–33
Børch K, Lou HC, Greisen G (2010) Cerebral white matter blood flow and arterial blood pressure in preterm infants. Acta Paediatr 99:1489–1492
Meek JH, Tyszczuk L, Elwell CE, Wyatt JS (1998) Cerebral blood flow increases over the first three days of life in extremely preterm neonates. Arch Dis Child Fetal Neonatal Ed 78:F33–F37
Pellicer A, Valverde E, Gayá F et al (2001) Postnatal adaptation of brain circulation in preterm infants. Pediatr Neurol 24:103–109
Kehrer M, Blumenstock G, Ehehalt S et al (2005) Development of cerebral blood flow volume in preterm neonates during the first two weeks of life. Pediatr Res 58:927–930
Freeman MR (2010) Specification and morphogenesis of astrocytes. Sxcience 330:774–778
Zecevic N (2004) Specific characteristic of radial glia in the human fetal telencephalon. Glia 48:27–35
Bushong EA, Martone ME, Ellisman MH (2004) Maturation of astrocyte morphology and the establishment of astrocyte domains during postnatal hippocampal development. Int J Devl Neuroscience 22:73–86
Sofroniew MV, Vinters HV (2010) Astrocytes: biology and pathology. Acta Neuropathol 119:7–35
Chaboub LS, Deneen B (2012) Developmental origins od astrocyte heterogeneity: the final frontier of CNS development. Dev Neurosci 34:379–388
Jakovcevski I, Filipovic R, Mo ZC et al (2009) Oligodendrocyte development and the onset of myelination in the human fetal brain. Front Neuroanat 3:5
Bradl M, Lassmann H (2010) Oligodendrocytes: biology and pathology. Acta Neuropathol 119:37–53
Back SA, Luo NL, Borenstein NS et al (2001) Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J Neurosci 21:1302–1312
Back SA, Luo NL, Borenstein NS et al (2002) Arrested oligodendrocytic lineage progression during human cerebral white matter development: dissociation between the timing of progenitor differentiation and myelinogenesis. J Neuropathol Exp Neurol 61:197–211
Flechsig P (1920) Anatomie des menschlichen Gehirns und Rückenmarks. Georg Thieme Verlag, Leipzig
Volpe JJ, Kinney HC, Jensen FE, Rosenberg PA (2011) The developing oligodendrocyte: key cellular target in brain injury in the premature infant. Int J Dev Neurosci 29:423–440
Buser JR, Maire J, Riddle A et al (2012) Arrested preoligodendrocyte maturation contributes to myelination failure in premature infants. Ann Neurol 71:93–109
Verney C, Pogledic I, Biran V et al (2012) Microglial reaction in axonal crossroads is a hallmark of non-cystic periventricular white matter injury in very preterm infants. J Neuropathol Exp Neurol 71:251–264
Rezaie P, Dean A, Male D, Ulfig N (2005) Microglia in the cerebral wall of the human telencephalon at second trimester. Cereb Cortex 15:938–949
Monier A, Evrard P, Gressens P, Verney C (2006) Distribution and differentiation of microglia in the human encephalon during the first two trimesters of gestation. J Comp Neurol 499:565–582
Verney C, Monier A, Fallet-Bianco C, Gressens P (2010) Early microglial colonization of the human forebrain and possible involvement in periventricular white-matter injury of preterm infants. J Anat 217:436–448
Judas M, Rados M, Jovanov-Milosevic N et al (2005) Structural, immunocytochemical, and MR imaging properties of periventricular crossroads of growing cortical pathways in preterm infants. Am J Neuroradiol AJNR 26:2671–2684
Volpe JJ (1989) Intraventricular hemorrhage in the premature infant: current concepts. Ann Neurol 25:3–11
Ghazi-Birry HS, Brown WR, Moody DM et al (1997) Human germinal matrix: venous origin of hemorrhage and vascular characteristics. AJNR Am J Neuroradiol 18:219–229
Ballabh P (2010) Intraventricular hemorrhage in premature infants: mechanism of disease. Pediatr Res 67:1–8
Tsitouras V, Sgouros S (2011) Infantile post hemorrhagic hydrocephalus. Childs Nerv Syst 27:1595–1608
Jary S, De Carli A, Ramenghi LA, Whitelaw A (2012) Impaired brain growth and neurodevelopment in preterm infants with posthaemorrhagic ventricular dilatation. Acta Paediatr 101:743–8
Vasileiadis GT, Gelman N, Han VKM et al (2004) Uncomplicated intraventricular hemorrhage is followed by reduced cortical volume at near-term age. Pediatrics 114:e367–e372
Bulat M, Klarica M (2011) Recent insight into a new hydrodynamics of the cerebrospinal fluid. Brain Res Rev 65:99–112
Filippidis AS, Kalani MY, Rekate HL (2011) Hydrocephalus and aquaporins: lessons learned from the bench. Childs Nerv Syst 27:27–33
Del Bigio MR (1993) Neuropathological changes caused by hydrocephalus. Acta Neuropathol 85:573–585
Takashima S, Mito T, Ando Y (1986) Pathogenesis of periventricular white matter hemorrhages in preterm infants. Brain Dev 8:25–30
Gould SJ, Howard S, Hope PL, Reynolds EOR (1987) Periventricular intraparenchymal cerebral haemorrhage in preterm infants: the role of venous infarction. J Pathol 151:197–202
Counsell SJ, Maalouf EF, Rutherford MA, Edwards AD (1999) Periventricular haemorrhagic infarct in a preterm neonate. Eur J Paediatr Neurol 3:25–28
De Vries LS, Roelants-van Rijn AM, Rademaker KJ et al (2001) Unilateral parenchymal haemorrhagic infarction in the preterm infant. Eur J Paediatr Neurol 5:139–149
Levene MI, de Vries LS (1984) Extension of neonatal intraventricular hemorrhage. Arch Dis Child 59:631–636
Rushton DI, Preston PR, Durbin GM (1985) Structure and evolution of echo dense lesions in the neonatal brain. A combined ultrasound and necropsy study. Arch Dis Child 60:798–808
Harteman JC, Nikkels PGJ, Kwee A et al (2012) Pattern of placental pathology in preterm infants with a periventricular haemorrhagic infarction: association with time of onset and clinical presentation. Placenta 33:839–844
Wolf BS, Huang YP (1964) The subependymal veins of the lateral ventricle. AJR Am J Roentgenol 90:474–489
Dudink J, Lequin M, Weisglas-Kuperus N et al (2008) Venous subtypes of preterm periventricular haemorrhagic infarction. Arch Dis Child Fetal Neonatal Ed 93:F201–F206
Arrigoni F, Parazzini C, Righini A et al (2011) Deep medullary vein involvement in neonates with brain damage: an MR imaging study. AJNR Am J Neuroradiol 32:2030–2036
Maitre NL, Marshall DD, Price WA et al (2009) Neurodevelopmental outcome in infants with unilateral or bilateral periventricular hemorrhagic infarction. Pediatrics 124:e1153–e1160
Staudt M (2010) Reorganization after pre- and perinatal brain lesions. J Anat 217:469–474
Marin-Padilla M (1996) Developmental neuropathology and impact of perinatal brain damage. I: Hemorrhagic lesions of neocortex. J Neuropathol Exp Neurol 55:758–773
Marin-Padilla M (1997) Developmental neuropathology and impact of perinatal brain damage. II: White matter lesions of the cortex. J Neuropathol Exp Neurol 56:219–235
Blümcke I, Thom M, Aronica E et al (2011) The clinicopathologic spectrum of focal cortical dysplasias: a consensus classification proposed by an ad hoc Task Force of the ILAE Diagnostic Methods Commission. Epilepsia 52:158–174
Keeney SE, Adcock EW, McArdle CB (1991) Prospective observations of 100 high-risk neonates by high-field (1.5 Tesla) manetic resonance imaging of the central nervous system. II. Lesions associated with hypoxic-ischemic encephalopathy. Pediatrics 87:431–438
Baenziger O, Martin E, Steinlin M et al (1993) Early pattern recognition in severe perinatal asphyxia: a prospective MRI study. Neuroradiology 35:437–442
Childs AM, Cornette L, Ramenghi L et al (2001) Magnetic resonance and cranial ultrasound characteristics of periventricular white matter abnormalities in newborn infants. Clin Radiol 56:647–655
Cornette LG, Tanner SF, Ramenghi LA et al (2002) Magnetic resonance of the infant brain: anatomical characteristics and clinical significance of the punctate lesions. Arch Dis Child Fetal Neonatal Ed 86:F171–F177
Ramenghi LA, Fumagalli M, Righini A et al (2007) Magnetic resonance imaging assessment of brain maturation in preterm neonates with punctate white matter lesions. Neuroradiology 49:161–167
Li AM, Chau V, Poskitt KJ et al (2009) White matter injury in term newborn with neonatal encephalopathy. Pediatr Res 65:85–89
Bassi L, Chew A, Merchant N et al (2011) Diffusion tensor imaging in preterm infants with punctate white matter lesions. Pediatr Res 69:561–566
Kato T, Okumura A, Tsuji T et al (2012) Punctate white matter lesions in a late preterm-born infant with hypoxic-ischaemic encephalopathy: chronological changes in magnetic resonance imaging. Dev Med Child Neurol 54:862
Kaur C, Ling EA (2009) Periventricular white matter damage in the hypoxic neonatal brain: role of microglial cells. Prog Neurobiol 87:264–280
Graeber MB (2010) Changing face of microglia. Science 330:783–788
Billiards SS, Haynes RL, Folkerth RD et al (2006) Development of microglia in the cerebral white matter of the human fetus and infant. J Comp Neurol 497:199–208
Hristova M, Cuthill D, Zbarsky V et al (2010) Activation and deactivation of periventricular white matter phagocytes during postnatal mouse development. Glia 58:11–28
Banker BQ, Larroche JC (1962) Periventricular leukomalacia of infancy. Arch Neurol 7:386–410
Friede RL (1975) Developmental neuropathology. Springer, Wien
Golden JA, Gilles FH, Rudelli R, Leviton A (1997) Frequency of neuropathological abnormalities in very low birth weight infants. J Neuropathol Exp Neurol 56:472–478
Volpe JJ (2001) Neurology of the newborn. Saunders, Philadelphia
Riddle A, Maire J, Gong X et al (2012) Differential susceptibility to axonopathy in necrotic and non-necrotic perinatal white matter injury. Stroke 43:178–184
McQuillen PS, Sheldon RA, Shatz CJ, Ferreiro DM (2003) Selective vulnerability of subplate neurons after early neonatal hypoxia-ischemia. J Neurosci 23:3308–3315
Kinney HC, Haynes RL, Xu G et al (2012) Neuron deficit in the white matter and subplate in periventricular leukomalacia. Ann Neurol 71:397–406
Leviton A, Gressens P (2007) Neuronal damage accompanies perinatal white-matter damage. TINS Trends Neurosci 30:473–478
Pierson CR, Folkerth RD, Billiards SS et al (2007) Gray matter injury associated with periventricular leukomalacia in the premature infant. Acta Neuropathol 114:619–631
Benders MJNL, Groenendaal F, Uiterwaal CSPM, de Vries LS (2008) Perinatal arterial stroke in the preterm infant. Semin Perinatol 32:344–349
Golomb MR, Garg BP, Edwards-Brown M, Williams LS (2008) Very early arterial ischemic stroke in premature infants. Pediatr Neurol 38:329–334
Ahdab-Barmada M, Moossy J, Painter M (1980) Pontosubicular necrosis and hyperoxemia. Pediatrics 66:840–847
Takashima S, Itoh M, Oka A (2009) A history of our understanding of cerebral vascular development and pathogenesis of perinatal brain damage over the past 30 years. Semin Pediatr Neurol 16:226–236
Acknowledgments
The authors thank John Wiley and Sons for permission to reproduce Fig. 5A from Okudera et al. Microangiographical studies of the medullary venous system of the cerebral hemisphere. Neuropathology 1999, 19:93–111 (reference 52 here).
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the special supplement “The Premature Brain"—Guest Editor: Charles Raybaud
Rights and permissions
About this article
Cite this article
Raybaud, C., Ahmad, T., Rastegar, N. et al. The premature brain: developmental and lesional anatomy. Neuroradiology 55 (Suppl 2), 23–40 (2013). https://doi.org/10.1007/s00234-013-1231-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-013-1231-0