Abstract
Purpose of Review
Coronavirus disease 2019 (COVID-19) pandemic has major health and economic impacts. We review disease characteristics in children.
Recent Findings
Children comprise 1–2% of the diagnosed cases, and typically suffer mild disease. The median age of infected children is 3.3–11 years, and male/female ratio is 1.15–1.55. Common symptoms in children include upper respiratory symptoms (26–54%), cough (44–54%), fever (32–65%), and gastrointestinal (15–30%) symptoms. Substantial proportion (4–23%) are asymptomatic. Death rates are up to 0.7%. Risk factors associated with severe disease are neonatal age group, male gender, lower respiratory tract disease, and pre-existing medical conditions. Vertical transmission was reported. Multisystem inflammatory syndrome (MIS), characterized by fever, multisystem organ involvement, and laboratory markers of inflammation, causes critical illness in > 50% of cases and is increasingly reported from endemic countries. Indirect effects of the coronavirus epidemic include higher rates of psychiatric morbidities, education loss, unhealthy lifestyle changes, and increased child neglect. Vaccines are in clinical trials and immunogenicity has not yet been shown in children.
Summary
Overall, COVID-19 has lower incidence and causes milder disease in children compared with adult patients. MIS is a rare severe complication more common in children. More data on the efficacy and safety of antivirals in children are needed.
Similar content being viewed by others
Introduction
Emerging in late 2019, coronavirus disease 2019 (COVID-19) has been spreading worldwide, with major health and economic impacts. By mid-August 2020, the World Health Organization reported over 23 million confirmed cases of infection with SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2), resulting in more than 710,000 death worldwide [1]. According to current data, children show lower incidence of symptomatic disease and develop a milder course [2,3,4,5]. We review the current evidence of epidemiology, clinical presentation, treatment, and indirect health consequences of SARS-CoV-2 on children.
Data Collection
We searched PubMed databases for publications using the search terms: “coronavirus 2019” OR “COVID-19” OR “SARS-CoV-2” OR “Novel Coronavirus” AND “children” OR “pediatric” OR “clinical trials” and for “Multisystem Inflammatory Syndrome in Children.” Additionally, we screened webpages and official guidelines of the US National Institutes of Health (NIH), Centers for Disease Control and Prevention (CDC), the World Health Organization (WHO), and the Infectious Diseases Society of America (IDSA) and Pediatric Infectious Disease Society (PIDS) for references. The search was restricted to English language publications during January–August 2020.
Pathophysiology
SARS-CoV-2 is an enveloped, positive sense single-stranded RNA virus with a glycoprotein spike (S) on the surface. Cell entry requires binding of the S protein to the cellular receptor ACE-2 (angiotensin-converting–enzyme-2) and priming of the S glycoprotein by the host cell serine protease TMPRSS2 [6]. The milder morbidity in children, despite similar or higher viral loads compared with adults [7, 8], is the focus of multiple studies but has yet to be fully understood. The differences may be partly explained by several characteristics of the pediatric immune system. According to the hypothesis by Carsetti et al., the immune system of children is highly prepared to novel pathogens, due to high levels of innate IgM antibodies and the ability to rapidly produce natural antibodies with broad reactivity, in addition to the production of the antiinflammatory interleukin (IL)-10 by neonatal B cells [9]. Additional suggested explanations are alterations in T cell populations in adults due to continuous antigen stimulation and thymic involution, varied levels of ACE-2 expression in children, and the simultaneous presence of other viruses in the respiratory mucosa of children, competing with SARS-CoV-2 [10]. Furthermore, children have fewer comorbidities and a stronger pulmonary regenerative potential than adults [11].
Epidemiology
Disease burden of COVID-19 in children is difficult to determine because case definitions for screening, testing, and disease severity in children are not universal and the proportion of asymptomatic infected children is high. In addition, young children attending daycare may contract several febrile and respiratory illnesses in a course of a few months [12], and it is plausible that SARS-CoV-2 test is not routinely performed.
In reports from countries that were severely affected early in course of the pandemic, children comprise 1–2% the diagnosed COVID-19 cases, underrepresented compared with other age groups [3, 13,14,15]. The median age of the diagnosed children ranges from 3.3–11 years in different reports, and data shows that children younger than 1 year are disproportionally represented [2, 3, 15,16,17]. Like in the adult population, there is a male predominance [2, 3, 15,16,17] (Table 1).
The contribution of children in spreading the virus through the community is a field of uncertainty, mainly due to the high rates of asymptomatic infection at younger age groups. A recent report found that in only 8% of households of 40 sick children, the child was the suspected index case. In the reminder, the child developed the symptoms following or together with a sick adult [18]. The same finding was seen in a cohort of sick children from China [19]. However, these cohorts may have been evaluated when educational institutes were closed, so children were less likely to contract the disease outside the house. In addition, recent reports show outbreaks in a high school and a summer camp [20, 21]. This is an issue of concern considering recent evidence of equivalent or higher amounts of viral nucleic acid in children < 5 years with mild to moderate disease, compared with older children and adults [8]. As schools worldwide are set to reopen, proposed adjustments of the education system include universal masking, breaking classes into capsules, attendance on alternate days, outdoors classrooms, online lessons, temperature checks, and reconfiguration of ventilation and air conditioning systems [22, 23].
Clinical Presentation and Disease Course
The rate of asymptomatic children, ranging from 4.4–23% of confirmed cases, is higher than reported in adults and most probably represents a significant underestimation as many asymptomatic children are not screened [2, 3, 16, 17].
The clinical presentation in adults ranges from mild illness to severe pneumonia. Severe cases may suffer complications including acute respiratory distress syndrome (ARDS), acute cardiac injury, and thromboembolic complications. Patients with severe disease have evidence of hyperimmune response with persistent fevers, elevated inflammatory markers (D-dimer, ferritin), and elevated proinflammatory cytokines [24, 25].
In children, respiratory symptoms are the most common, followed by fever and gastrointestinal symptoms [3, 16,17,18] (Table 1). Anosmia and ageusia are commonly described in adults [26] but may be more difficult to elicit in young children and thus underreported [27].
The rate of children with critical illness ranges from 0.4–9% of confirmed cases, probably reflecting population bias since some reports include mainly patients diagnosed in hospitals [3, 4, 15,16,17]. Data from the USA indicates a hospitalization rate of 8 per 100,000 population in children < 18 years, much lower than 164.5 per 100,000 in adults. However, a third of the hospitalized children required admission to the intensive care unit (ICU) [28].
The analysis of critical patients may indicate which children are at higher risk. In various reports, half of the children admitted to the ICU had an underlying medical condition [15,16,17, 28, 29]. Factors associated with ICU admissions were neonatal age group, male gender, lower respiratory tract disease, and pre-existing medical conditions [17]. Infants aged < 3 months comprised 19% of the hospitalized children in a recent report from the USA. However, this may have been due to the diagnosis of neonatal fever and not due to disease severity [28]. Because these data are driven from potentially biased datasets with over representation of symptomatic children, the association of young age and severity needs to be further investigated.
Death ensued in 0–0.7% of diagnosed children [2, 3, 14,15,16]. In the European cohort of 582 children aged 0–18 years, four children died, all were older than 10 years, two had no pre-existing medical conditions, one had undergone human stem cell transplant (HSCT) 15 months earlier, and the other patient’s condition was not specified [17]. In a multinational study from North America on children hospitalized in the ICU, two patients, aged 12 and 17 years, died; both had unspecified pre-existing comorbidities and 1 had also prior gram-negative sepsis [29]. In a cohort from New York, one patient with metastatic malignancy died [30]. Two cohorts from China reported one death each—a 10-month-old baby with intussusception [16] and a 14-year-old boy with no further details [2]. Because mortality rates in pediatric cohorts are low, it is difficult to define risk factors and disease course leading to fatal result in pediatric population.
A more distinct population among the pediatric population are neonates. Early reports showed that infants of mothers infected in the last trimester have normal course and that amniotic fluid, umbilical cord blood, throat swab, and breast milk were negative to the virus [31, 32]. However, more recent reports show clinical infections in neonates and suggest prevention procedures, such as isolation of the infant and physical barriers after the delivery [33,34,35]. Recently, vertical transmission with high placental viral load and neonatal compromise requiring resuscitation was documented [36]. When screening neonates, a possible contamination of the neonatal swab by maternal SARS-CoV-2 should be taken into account [37], in addition to transmission of an undiagnosed maternal infection after birth.
In a review of cases in the neonatal period, most of them were asymptomatic (20%) or had mild (48%) and moderate (20%) signs of clinical infection. The rate of severely ill patients was higher compared with older children (12% vs. up to 9% in the general pediatric population aged < 18 years). Dyspnea was the most common sign (40%), followed by fever (32%) and feeding intolerance (24%) [4].
Multisystem Inflammatory Syndrome—Children
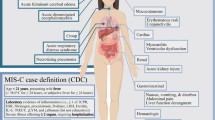
Since May 2020, several highly endemic countries reported an exceptional high incidence of multisystem inflammatory syndrome (MIS) in children [38,39,40,41,42,43]. Several case definitions were proposed, all include fever, elevated inflammatory markers, and organ dysfunction not attributed to another infectious cause (Table 2). A minority of patients had been symptomatic prior to onset of MIS onset, and the median interval from COVID-19 symptom onset to MIS onset is 25 days [44]. The higher rate of positive serologic tests compared with nasopharyngeal reverse transcription–polymerase chain reaction (RT-PCR) is suggestive of a late complication of the disease (Table 3) [38, 39, 41, 44, 45]. According to the Centers for Disease Control and prevention (CDC) report, in 27% of the patients, both were positive. Similar to the acute COVID-19 [30], obesity is a risk factor for MIS, present in a quarter of the patients in the CDC report [43]. Suggested mechanisms for MIS include viral mimicry, formation of immune complexes, and host immune cell activation due to viral superantigen sequences [46].
Besides fever, the most common presentations of MIS are gastrointestinal (diarrhea, vomiting, abdominal pain), cardiovascular, mucocutaneous (rash, mucus membrane changes, conjunctival injection), respiratory (including sore throat), headache, and limb and periorbital edema [39, 43, 44]. Associated laboratory findings are elevated inflammation markers (neutrophilia, C-reactive protein, ferritin, erythrocyte sedimentation rate), thrombocytopenia, lymphopenia, elevated troponin and N-terminal pro-B-type natriuretic peptide (NT-proBNP), hypertriglyceridemia, and elevated D-dimer and fibrinogen. Some patients meet the criteria for macrophage activation syndrome (MAS).
The disease course is typically severe, with high rates of ICU admissions, mechanical ventilation, and death (Table 3). Severe course is characterized by shock, and coronary aneurysms [39, 41, 43, 44, 47, 48]. In addition to supportive care, children diagnosed with MIS were treated with intravenous immune globulin ± aspirin, glucocorticoids, IL-6 receptor antagonist, IL-1 receptor antagonist, and TNF-α antagonist [48]. In the larger cohorts, from the USA, 2% of the patients died, one of them reported that half of them had underlying medical conditions [43, 44].
The clinical presentation was compared with that of Kawasaki disease. However, children diagnosed with MIS were older [38, 39, 44, 47], showed a greater elevation of inflammatory markers [39], and more frequent cardiovascular hemodynamic involvement [38, 44, 47].
Treatment
Treatment in children infected with SARS-CoV-2 consists mainly on supportive care, including oxygen and advanced respiratory support, hydration, and antipyretics [49, 50]. Metered dose inhalers are preferred over nebulizers due to the decreased risk of virus dissemination [49, 51].
The inclusion of children in early phase clinical trials of novel agents is usually delayed; hence, trial data regarding efficacy and safety are scarce [52].
Antiviral and antiinflammatory drugs may be considered in severely ill children and those at higher risk for severe disease, preferably as part of a clinical trial. Severe disease is often associated with hyperinflammation and cytokine storm that may lead to acute respiratory distress syndrome [49, 53]. Hence, medications targeted to the immune system were suggested, in addition to antivirals. Several medications were used in hospitalized children [28, 29], but not in the context of a clinical trial, making it difficult to accurately assess their outcome.
The antiviral agent remdesivir was suggested as the preferred agent for treating COVID-19 in children [50]. This is an adenine nucleoside analogue that interferes with the virus’ RNA-dependent RNA polymerase. Remdesivir was used in children suffering Ebola infection, but pediatric safety data were not separately reported [54]. Studies in adults showed it may have some benefit [55,56,57], and clinical trials including children are ongoing. Currently, the NIH recommends using remdesivir in adults with severe disease [58]. The drug is available through the US Food and Drug Administration Emergency Use Authorization and compassionate use requests are reviewed by the manufacturer [59]. A clinical trial is currently evaluating the pharmacokinetics in children [58].
Dexamethasone was found in the randomized-controlled UK-based RECOVERY trial to reduce mortality in patients who require respiratory support [60]. Despite the enrolment of children, it is not clear if the analysis included children, so further data is still needed. The NIH guidelines state that dexamethasone may be beneficial in pediatric patients who require mechanical ventilation and suggest treatment according to individual considerations in milder cases [58]. Another potential adjunctive therapy for COVID-19 is convalescent plasma, with only scarce experience in adults [58, 61, 62]. Shekerdemian et al. reported the use of convalescent plasma in a child, but the results were not discussed [29]. Currently, there are insufficient data to recommend either for or against the use of convalescent plasma for the treatment of COVID-19. Clinical trials of COVID-19 convalescent plasma in children are ongoing [58].
Other drugs were initially suggested for the treatment of COVID-19 and their use in infected children was reported [28, 29]. However, current recommendations are against their use due to questionable safety and efficacy [58]. The antiviral lopinavir/ritonavir (Kaletra) is a protease inhibitor used for treatment of HIV infection, including young infants. Its suggested mechanism of action is inhibition of the SARS-CoV-2 proteinases papain-like proteinase and 3C-like proteinase, which are key enzymes in polyprotein processing [50]. The NIH recommends against its use outside of clinical trials in COVID-19 due to lack of proven efficacy and concerns on its pharmacodynamics [63]. Hydroxychloroquine was suggested as another potential treatment. It was previously shown to inhibit SARS-CoV-2 entry into cells and interfere with the glycosylation of the ACE-2 receptor (virus’ binding site) and inhibit its spread [64, 65], and has additional host immunomodulatory effects [61]. There is no solid evidence for its efficacy in adults [66]. The drug is available and was previously used to treat children in other indications. Due to substantial risk of QT prolongation, it is not recommended combining hydroxychloroquine with azithromycin. Patients with known G6PD deficiencies should be monitored for hemolysis [50]. Shekerdemian et al. reported its use in almost half of a cohort of children hospitalized in intensive care units in North America, but there is no analysis of the outcome of the specific treatment [24]. The NIH recommends against its use except for clinical trials [58]. Tocilizumab, an IL-6 receptor antagonist, was used in adults with cytokine storm and hyperinflammation due to SARS-CoV-2 with conflicting results [53, 67,68,69], and in a small number of children admitted to ICU, but the outcome was not specified [29]. It is FDA approved to treat cytokine release syndrome in children 2 years of age and older, and in the RECOVERY trial used in children > 1 year [52, 70]. Screening and monitoring infectious complications especially latent tuberculosis should be performed prior and during therapy [69]. The NIH recently recommended against the use of IL-6 inhibitors for the treatment of COVID-19, except for clinical trials [58]. However, reports suggest its use in children who develop multisystem inflammatory syndrome (discussed later).
Other potential treatments are currently under evaluation, including antiIL-1 (anakinra), interferon-beta, and ivermectin [49, 58, 61].
Special Populations
Following the concern regarding the consequences of SARS-CoV-2 infection in children with chronic diseases, several guidelines were published.
A statement endorsed by the US Pediatric Infectious Diseases Society has recently proposed that children with severe immunocompromise, severe cardiac, or severe pulmonary diseases may be more likely to experience severe COVID-19 disease. Obesity and diabetes should be also taken into account, especially with comorbidities [50].
The European Academy of Allergy and Clinical Immunology recommends treating children with allergic asthma, allergic rhinitis, or other allergy conditions according to usual guidelines. One exception to this is the advice to withhold biologics (antiIL-5Rα, IL-4Rα, and omalizumab) during acute COVID-19 disease, since they are directed towards type 2 response, which may counteract the “cytokine storm” seen in severe COVID-19 [71, 72]. The Global Initiative for Asthma (GINA) recommends continuation of inhaled asthma treatment and treatment with biologic therapies if needed. Treatment with oral corticosteroids should be administered in the lowest possible dose in patients at risk of severe attacks [51].
Patients with immunodeficiency, either primary, secondary to other diseases or medical treatments, are advised to strictly follow national precaution recommendations and in case of a suspected infection be in touch with their physician [71]. Clear data regarding the severity of the disease in immunocompromised children are lacking. Previously, immunocompromised children showed increased risk for severe lower respiratory tract disease due to seasonal coronaviruses [73•]. On the contrary, a report from Bergamo, Italy, stated that children who underwent liver transplant did not develop clinical pulmonary disease during the outbreak [74]. In adults, patients with malignancy and solid organ transplant recipients may be at increased risk of severe COVID-19 disease and death. Evidence regarding other types of immunocompromise is scarce [75]. According to the guidance endorsed by the pediatric infectious diseases experts, patients with mild to moderate immunodeficiency were not proven to be at increased risk, and those severely immunocompromised (e.g., severe combined immunodeficiency, < 100 days post-allogenic-HSCT, HIV infection with CD4 count < 15% or < 200/mm3, treatment with costimulation inhibitors like belatacept or abatacept, high-dose corticosteroids, and more conditions) should be considered for antiviral treatment. The panel suggests reducing T cell immunosuppression in infected children [50].
Indirect Consequences on Child Health
Beyond the physiological manifestation of COVID-19, other pediatric health issues during this pandemic bear mention.
Data show that lockdown, combined with intense fear of COVID-19 contagion, led to a dramatic decrease in patients seeking medical care for other emergent issues [76,77,78]. In addition, ambulatory and screening services were postponed, including routine immunizations given to infants and children [79]. Lower immunization rates may diminish herd immunity for some vaccine preventable diseases and lead to the re-emergence of other infectious diseases in children. This trend may wane as the epidemic continues and routine health seeking behaviors resume. Masking of the medical staff poses another barrier for the routine medical care of children, making communication with pediatric patients challenging [80].
School closure was a major step of infection control in many countries, affecting over 1.6 billion learners [81]. The consequences to the child’s well-being of these steps are numerous: learning loss, (especially for those in low-income settings), lack of access to school-provided social assistance, reduced physical activity, and a significant harm to social life. In low- and middle-income countries, where access to education may be limited, some children may drop out as a result of the indirect impact of the outbreak [81, 82].
Following school reopening, frontal teaching is partially replaced by remote online lessons. Despite its innovative nature, this mode of studying is impossible to children affected by a lack of resources and requires extreme effort from children dealing with attention deficit hyperactivity disorder (ADHD) [83]. A study from China found that children’s ADHD behaviors significantly worsened during COVID-19 outbreak in comparison with their normal state [84]. The European ADHD guidance group (EAGG) adjusted its protocol, and frontal cardiovascular exam is no longer needed to initiate drug therapy, given normal personal and familial cardiac history and normal blood pressure and heart rate [85]. Additional effects of the epidemic on mental health include anxiety and depression [86,87,88]. A survey among Chinese school-aged children during lockdown revealed higher rates of anxiety and depression than usual [89].
Safe, secure, and supportive domestic environment for children requires engaged parenting. However, during these times, parents are challenged by unemployment, remote work, economic instability, home confinement, health worries, and home-learning of their children [90, 91]. Thus, children are at higher risk than usual to neglect abuse and domestic violence [91,92,93]. Despite increased incidence of child abuse and neglect during COVID-19 pandemic [93, 94], the number of official reports to maltreatment lines in a few US states decreased sharply, raising a concern of under-reporting due to decreased contact with the insulted children. Spotting signs for abuse and assessing home safety through distance learning should be practiced [93].
The economic impact of the pandemic is likely to deepen unemployment and poverty worldwide. The resultant food insecurity and malnutrition are concerning [95, 96], particularly in young children who are the most vulnerable to its consequences [97].
On the other hand, in wealthier countries, quarantine, social distancing, and parental difficulties led to unhealthy lifestyle modifications among adolescents with increased consumption of unhealthy foods and reduction in physical activity that may lead to obesity and sleep disorders [98,99,100,101,102]. Suggested steps to encourage physical activity during this period include incorporating physical activity into children’s daily routine, using electronic devices for engaging children to physical activity, encouraging family members to join ongoing activities, and avoiding extended sitting [101].
Prevention
The standard precautions face masks, hand hygiene, and social distancing are extremely difficult to implement in young children. Alcohol-based hand sanitizers contain above 60% ethanol, and according to the CDC should be used with adult supervision in children under 6 years of age. The use of masks may be cumbersome in children. The minimal proposed age for mask use is 2 years old. In younger ages, the smaller airways may interfere with breathing and the child may be unable to remove the mask on his own. In older children, size fitted mask and education on appropriate mask removal are needed [103].
Over 140 SARS-CoV-2 vaccine candidates are currently evaluated, including nucleic acid–based, viral vector vaccines, and inactivated or recombinant protein vaccines. Most of them focus on immunity against the viral spike (S) glycoprotein [104, 105]. Results of three vaccine trials were recently published: a phase 1 trial of an mRNA vaccine that encodes the S glycoprotein [105], a phase 1 trial of a recombinant adenovirus type-5 vectored expressing the S glycoprotein [106], and a phase 1/2 trial of a chimpanzee adenovirus–vectored vaccine expressing the S glycoprotein (ChAdOx1 nCoV-19) [107]. All showed both humoral and cellular immunogenicity to the spike glycoprotein. The most common reported side effects include fatigue, headache, and fever, with higher rates compared with other vaccines [105,106,107]. None of the trials included children.
Conclusions
In summary, children at any age may be infected with SARS-CoV-2, with reduced frequency and severity compared with adults, although clear epidemiologic data is still missing. In addition, the recently identified MIS may pose an additional threat. Data on the outcome of antiviral treatments, the safety and immunogenicity of vaccinations, and better specification of high-risk patients in the pediatric population are still needed. As the pandemic continues to evolve, it is still hard to fully assess or forecast the mid- and long-term effects of the resulting significant changes to society, economics, and human behavior on future child health and well-being. It is important that both medical and social efforts focusing on the pediatric population are undertaken to protect the children of the world allowing them to fulfill their enormous potential.
References
No Title [Internet]. WHO Coronavirus Dis. Dashboard. [cited 2020 Jul 18]. Available from: covid19.who.int/.
Dong Y, Mo X, Hu Y, Qi X, Jiang F, Jiang Z. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145.
Bialek S, Gierke R, Hughes M, McNamara LA, Pilishvili T, Skoff T. Coronavirus disease 2019 in children — United States, February 12–April 2, 2020. Morb Mortal Wkly Rep [Internet]. Department of Health and Human Services; 2020 [cited 2020 Jul 3];69:422–6. Available from: https://www.cdc.gov/mmwr/volumes/69/wr/mm6914e4.htm.
Liguoro I, Pilotto C, Bonanni M, Ferrari ME, Pusiol A, Nocerino A, et al. SARS-COV-2 infection in children and newborns : a systematic review. Eur J Pediatr. 2020;179:1029–46.
Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults [Internet]. Acta Paediatr. Int. J. Paediatr. Blackwell Publishing Ltd; 2020 [cited 2020 Jul 18]. p. 1088–95. Available from: https://doi.org/10.1111/apa.15270.
Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell [Internet]. Cell Press; 2020 [cited 2020 Jul 31];181:271–280.e8. Available from: https://pubmed-ncbi-nlm-nih-gov.sheba-ez.medlcp.tau.ac.il/32142651/.
Walsh KA, Jordan K, Clyne B, Rohde D, Drummond L, Byrne P, et al. SARS-CoV-2 detection, viral load and infectivity over the course of an infection. J Infect [Internet]. Elsevier; 2020 [cited 2020 Jul 18];0. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0163445320304497.
Heald-Sargent T, Muller WJ, Zheng X, Rippe J, Patel AB, Kociolek LK. Age-related differences in nasopharyngeal severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) levels in patients with mild to moderate coronavirus disease 2019 (COVID-19) [Internet]. JAMA Pediatr. American Medical Association; 2020 [cited 2020 Aug 25]. Available from: https://jamanetwork.com/.
Carsetti R, Quintarelli C, Quinti I, Piano Mortari E, Zumla A, Ippolito G, et al. The immune system of children: the key to understanding SARS-CoV-2 susceptibility? [Internet]. Lancet Child Adolesc. Heal. Elsevier B.V.; 2020 [cited 2020 Jul 11]. p. 414–6. Available from: https://www.psychiatry.
Yuki K, Fujiogi M, Koutsogiannaki S. COVID-19 pathophysiology: a review [Internet]. Clin Immunol. Academic Press Inc.; 2020 [cited 2020 Jul 31]. Available from: https://pubmed.ncbi.nlm.nih.gov/32325252/
Dhochak N, Singhal T, Kabra SK, Lodha R. Pathophysiology of COVID-19: Why children fare better than adults? 2098 [cited 2020 Aug 8]; Available from: https://doi.org/10.1007/s12098-020-03322-y.
Schuez-Havupalo L, Toivonen L, Karppinen S, Kaljonen A, Peltola V. Daycare attendance and respiratory tract infections: a prospective birth cohort study. BMJ Open [Internet]. BMJ Publishing Group; 2017 [cited 2020 Jul 19];7. Available from: /pmc/articles/PMC5588939/?report=abstract.
Surveillances V. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases ( COVID-19 ) — China, 2020. 2020;2:113–22.
Livingston E, Bucher K. Coronavirus disease 2019 (COVID-19) in Italy. JAMA [Internet]. NLM (Medline); 2020 [cited 2020 Jul 19];323:1335–1335. Available from: https://www.iss.it/infografiche
Parri N, Lenge M, Buonsenso D. Children with COVID-19 in Pediatric Emergency Departments in Italy. N Engl J Med [Internet]. Massachussetts Medical Society; 2020 [cited 2020 Aug 8];383:187–90. Available from: http://www.nejm.org/doi/10.1056/NEJMc2007617.
Lu X, Zhang L, Du H, Zhang J, Li YY, Qu J, et al. SARS-CoV-2 Infection in children. N Engl J Med [Internet]. Massachussetts Medical Society; 2020 [cited 2020 Jul 19];382:1663–5. Available from: http://www.nejm.org/doi/10.1056/NEJMc2005073.
Götzinger F, Santiago-garcía B, Noguera-julián A, Lanaspa M, Lancella L, Carducci FIC. Articles COVID-19 in children and adolescents in Europe : a multinational , multicentre cohort study. 2020;4642:1–9.
Posfay-Barbe KM, Wagner N, Gauthey M, Moussaoui D, Loevy N, Diana A, et al. COVID-19 in children and the dynamics of infection in families. Pediatrics [Internet]. American Academy of Pediatrics (AAP); 2020 [cited 2020 Jul 18];e20201576. Available from: https://doi.org/10.1542/peds.2020-1576.
Wu Q, Xing Y, Shi L, Li W, Gao Y, Pan S, et al. Coinfection and other clinical characteristics of COVID-19 in children. Pediatrics [Internet]. American Academy of Pediatrics (AAP); 2020 [cited 2020 Jul 18];146:e20200961. Available from: https://pubmed.ncbi.nlm.nih.gov/32376725/.
Szablewski CM, Chang KT, Brown MM, Chu VT, Yousaf AR, Anyalechi N, et al. SARS-CoV-2 transmission and infection among attendees of an overnight camp — Georgia, June 2020. MMWR Morb Mortal Wkly Rep [Internet]. 2020 [cited 2020 Aug 8];69:1023–5. Available from: http://www.cdc.gov/mmwr/volumes/69/wr/mm6931e1.htm?s_cid=mm6931e1_w.
Stein-Zamir C, Abramson N, Shoob H, Libal E, Bitan M, Cardash T, et al. A large COVID-19 outbreak in a high school 10 days after schools’ reopening, Israel, May 2020. Eurosurveillance [Internet]. European Centre for Disease Control and Prevention (ECDC); 2020 [cited 2020 Aug 8];25:2001352. Available from: https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2020.25.29.2001352.
Plan for school reopening | IIEP-UNESCO [Internet]. [cited 2020 Aug 29]. Available from: http://www.iiep.unesco.org/en/plan-school-reopening.
Vermund SH, Pitzer VE. Asymptomatic transmission and the infection fatality risk for COVID-19: implications for school reopening. Clin Infect Dis [Internet]. Oxford University Press (OUP); 2020 [cited 2020 Aug 29]; Available from: https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciaa855/5862668.
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet [Internet]. Lancet Publishing Group; 2020 [cited 2020 Aug 1];395:497–506. Available from: https://isaric.tghn.org/protocols/.
Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression [Internet]. Lancet. Lancet Publishing Group; 2020 [cited 2020 Aug 1]. p. 1033–4. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7270045/.
Vaira LA, Salzano G, Deiana G, De Riu G. Anosmia and ageusia: common findings in COVID-19 patients. Laryngoscope [Internet]. John Wiley and Sons Inc.; 2020 [cited 2020 Jul 3];130:1787. Available from: /pmc/articles/PMC7228304/?report=abstract.
Mak PQ, Chung K-S, Wong JS-C, Shek C-C, Kwan MY-W. Anosmia and ageusia: not an uncommon presentation of COVID-19 infection in children and adolescents. Pediatr Infect Dis J [Internet]. Lippincott Williams and Wilkins; 2020 [cited 2020 Aug 31];39:e199–200. Available from: https://doi.org/10.1097/INF.0000000000002718.
Kim L, Whitaker M, O’Halloran A, Kambhampati A, Chai SJ, Reingold A, et al. Hospitalization rates and characteristics of children aged <18 years hospitalized with laboratory-confirmed COVID-19 — COVID-NET, 14 States, March 1–July 25, 2020. MMWR Morb Mortal Wkly Rep [Internet]. Centers for Disease Control MMWR Office; 2020 [cited 2020 Aug 25];69:1081–8. Available from: http://www.cdc.gov/mmwr/volumes/69/wr/mm6932e3.htm?s_cid=mm6932e3_w.
Shekerdemian LS, Mahmood NR, Wolfe KK, Riggs BJ, Ross CE, McKiernan CA, et al. Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to US and Canadian Pediatric Intensive Care Units. JAMA Pediatr [Internet]. American Medical Association; 2020 [cited 2020 Jul 19]; Available from: https://jamanetwork.com/.
Chao JY, Derespina KR, Herold BC, Goldman DL, Aldrich M, Weingarten J, et al. Clinical characteristics and outcomes of hospitalized and critically ill children and adolescents with coronavirus disease 2019 at a tertiary care medical center in New York City. J Pediatr [internet]. Mosby Inc.; 2020 [cited 2020 Aug 26];223:14-19.e2. Available from: https://doi.org/10.1016/j.jpeds.2020.05.006.
Chen H, Guo J, Wang C, Luo F, Yu X, Zhang W, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet [Internet]. Lancet Publishing Group; 2020 [cited 2020 Jul 11];395:809–15. Available from: https://pubmed.ncbi.nlm.nih.gov/32151335/.
Shahbazi Sighaldeh S, Ebrahimi Kalan M. Care of newborns born to mothers with COVID-19 infection; a review of existing evidence. J Matern Fetal Neonatal Med [Internet]. NLM (Medline); 2020 [cited 2020 Jul 12];1–13. Available from: https://www.tandfonline.com/action/journalInformation?journalCode=ijmf20.
Zeng L, Xia S, Yuan W, Yan K, Xiao F, Shao J, et al. Neonatal early-onset infection with SARS-CoV-2 in 33 neonates born to mothers with COVID-19 in Wuhan, China [Internet]. JAMA Pediatr. American Medical Association; 2020 [cited 2020 Jul 12]. p. 800–4. Available from: https://jamanetwork.com/
Zaigham M, Andersson O. Maternal and perinatal outcomes with COVID-19: a systematic review of 108 pregnancies. Acta Obstet Gynecol Scand [Internet]. Wiley-Blackwell; 2020 [cited 2020 Jul 12];99:823–9. Available from: https://doi.org/10.1111/aogs.13867.
Chandrasekharan P, Vento M, Trevisanuto D, Partridge E, Underwood MA, Wiedeman J, et al. Neonatal resuscitation and postresuscitation care of infants born to mothers with suspected or confirmed SARS-CoV-2 infection. Am J Perinatol [Internet]. NLM (Medline); 2020 [cited 2020 Jul 12];37. Available from: https://pubmed.ncbi.nlm.nih.gov/32268381/.
Vivanti AJ, Vauloup-Fellous C, Prevot S, Zupan V, Suffee C, Do Cao J, et al. Transplacental transmission of SARS-CoV-2 infection. Nat Commun [Internet]. Nature Publishing Group; 2020 [cited 2020 Jul 25];11:3572. Available from: http://www.ncbi.nlm.nih.gov/pubmed/32665677.
Zeng L, Xia S, Yuan W, Yan K, Xiao F, Shao J, et al. Neonatal early-onset infection with SARS-CoV-2 in 33 neonates born to mothers with COVID-19 in Wuhan, China [Internet]. JAMA Pediatr. American Medical Association; 2020 [cited 2020 Jul 17]. Available from: https://pubmed.ncbi.nlm.nih.gov/32215598/.
Verdoni L, Mazza A, Gervasoni A, Martelli L, Ruggeri M, Ciuffreda M, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet [internet]. Elsevier ltd; 2020;395:1771–8. Available from: https://doi.org/10.1016/S0140-6736(20)31103-X.
Whittaker E, Bamford A, Kenny J, Kaforou M, Jones CE, Shah P, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA - J Am Med Assoc 2020;1–11.
Rauf A, Vijayan A, John ST, Krishnan R, Latheef A. Multisystem inflammatory syndrome with features of atypical Kawasaki disease during COVID-19 pandemic. Indian J Pediatr; 2020;2–4.
Toubiana J, Poirault C, Corsia A, Bajolle F, Fourgeaud J, Angoulvant F, et al. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ [Internet]. NLM (Medline); 2020 [cited 2020 Jul 4];369:m2094. Available from: https://doi.org/10.1136/bmj.m2094.
Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic [Internet]. Lancet. Lancet Publishing Group; 2020 [cited 2020 Jul 19]. p. 1607–8. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7204765/.
Godfred-Cato S, Bryant B, Leung J, Oster ME, Conklin L, Abrams J, et al. COVID-19–associated multisystem inflammatory syndrome in children — United States, March–July 2020. MMWR Morb Mortal Wkly Rep [Internet]. Centers for Disease Control MMWR Office; 2020 [cited 2020 Aug 25];69:1074–80. Available from: http://www.cdc.gov/mmwr/volumes/69/wr/mm6932e2.htm?s_cid=mm6932e2_w.
Feldstein LR, Rose EB, Horwitz SM, Collins JP, Newhams MM, Son MBF, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med [Internet]. Massachusetts Medical Society; 2020 [cited 2020 Jul 3];NEJMoa2021680. Available from: https://doi.org/10.1056/NEJMoa2021680.
Nakra NA, Blumberg DA, Herrera-Guerra A, Lakshminrusimha S. Multi-system inflammatory syndrome in children (MIS-C) following SARS-CoV-2 infection: review of clinical presentation, hypothetical pathogenesis, and proposed management. Child (Basel, Switzerland) [Internet]. Children (Basel); 2020 [cited 2020 Sep 5];7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/32630212.
Jiang L, Tang K, Levin M, Irfan O, Morris SK, Wilson K, et al. COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect Dis [Internet]. Elsevier BV; 2020 [cited 2020 Sep 5]; Available from: https://pubmed.ncbi.nlm.nih.gov/32818434/.
Belhadjer Z, Méot M, Bajolle F, Khraiche D, Legendre A, Abakka S, et al. Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic. Circulation [Internet]. Ovid Technologies (Wolters Kluwer Health); 2020 [cited 2020 Jul 3]; Available from: http://ahajournals.org.
Henderson LA, Canna SW, Friedman KG, Gorelik M, Lapidus SK, Bassiri H, et al. American College of Rheumatology Clinical Guidance for Pediatric Patients with Multisystem Inflammatory Syndrome in Children (MIS-C) associated with SARS-CoV-2 and hyperinflammation in COVID-19. Version 1. Arthritis Rheumatol [Internet]. Wiley; 2020 [cited 2020 Sep 4]; Available from: https://pubmed.ncbi.nlm.nih.gov/32705809/.
NHS England. Clinical management of persons admitted to hospital with suspected COVID-19 infection. 2020;1–10.
Chiotos K, Hayes M, Kimberlin DW, Jones SB, James SH, Pinninti SG, et al. Multicenter initial guidance on use of antivirals for children with COVID-19/SARS-CoV-2. J Pediatric Infect Dis Soc 2020;1–15.
COVID-19: GINA ANSWERS TO FREQUENTLY ASKED QUESTIONS ON ASTHMA MANAGEMENT [Internet]. [cited 2020 Jul 19]. Available from: https://ginasthma.org/covid-19-gina-answers-to-frequently-asked-questions-on-asthma-management/.
COVID-19 pharmacologic treatments for children: research priorities and approach to pediatric studies | Clin Infect Dis. | Oxford Academic [Internet]. [cited 2020 Jul 4]. Available from: https://doi.org/10.1093/cid/ciaa885/5864500.
Guaraldi G, Meschiari M, Cozzi-Lepri A, Milic J, Tonelli R, Menozzi M, et al. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol [Internet]. Elsevier BV; 2020 [cited 2020 Jul 11];0. Available from: www.thelancet.com/rheumatologyPublishedonline.
Mulangu S, Dodd LE, Davey RT, Tshiani Mbaya O, Proschan M, Mukadi D, et al. A Randomized, Controlled trial of Ebola virus disease therapeutics. N Engl J Med [Internet]. Massachussetts Medical Society; 2019 [cited 2020 Sep 1];381:2293–303. Available from: https://doi.org/10.1056/NEJMoa1910993.
Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet [Internet]. Lancet Publishing Group; 2020 [cited 2020 Jul 10];395:1569–78. Available from: https://isaric.tghn.org/.
Grein J, Ohmagari N, Shin D, Diaz G, Asperges E, Castagna A, et al. Compassionate use of remdesivir for patients with severe covid-19. N Engl J Med [Internet]. Massachussetts Medical Society; 2020 [cited 2020 Jul 10];382:2327–36. Available from: https://doi.org/10.1056/NEJMoa2007016.
Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of COVID-19 — preliminary report. N Engl J Med [Internet]. Massachusetts Medical Society; 2020 [cited 2020 Jul 11]; Available from: https://doi.org/10.1056/NEJMoa2007764.
COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. Available at https://www.covid19treatmentguidelines.nih.gov/. Accessed 2020 Sep 4.
Emergency access to remdesivir outside of clinical trials [Internet]. [cited 2020 Jul 19]. Available from: https://www.gilead.com/purpose/advancing-global-health/covid-19/emergency-access-to-remdesivir-outside-of-clinical-trials.
Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al. Dexamethasone in hospitalized patients with COVID-19 — preliminary report. N Engl J Med [Internet]. Massachusetts Medical Society; 2020 [cited 2020 Jul 19];NEJMoa2021436. Available from: https://doi.org/10.1056/NEJMoa2021436.
Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA - J Am Med Assoc. 2020;323:1824–36.
Duan K, Liu B, Li C, Zhang H, Yu T, Qu J, et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci U S A [Internet]. National Academy of Sciences; 2020 [cited 2020 Jul 11];117:9490–6. Available from: https://osf.io/gahz5.
Lopinavir/ritonavir and other HIV protease inhibitors | Coronavirus Disease COVID-19 [Internet]. [cited 2020 Jul 19]. Available from: https://www.covid19treatmentguidelines.nih.gov/antiviral-therapy/lopinavir-ritonavir-and-other-hiv-protease-inhibitors/.
Vincent MJ, Bergeron E, Benjannet S, Erickson BR, Rollin PE, Ksiazek TG, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. 2005 [cited 2020 Jul 11]; Available from: http://www.virologyj.com/content/2/1/69.
Liu J, Cao R, Xu M, Wang X, Zhang H, Hu H, et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov [Internet]. Springer Nature; 2020 [cited 2020 Jul 11];6:16. Available from: http://www.nature.com/articles/s41421-020-0156-0.
Chloroquine or hydroxychloroquine | Coronavirus Disease COVID-19 [Internet]. [cited 2020 Jul 19]. Available from: https://www.covid19treatmentguidelines.nih.gov/antiviral-therapy/chloroquine-or-hydroxychloroquine/.
Zhang C, Wu Z, Li JW, Zhao H, Wang GQ. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents [Internet]. Elsevier B.V.; 2020 [cited 2020 Jul 11];55:105954. Available from: /pmc/articles/PMC7118634/?report=abstract.
Colaneri M, Bogliolo L, Valsecchi P, Sacchi P, Zuccaro V, Brandolino F, et al. Tocilizumab for treatment of severe COVID-19 patients: preliminary results from SMAtteo COvid19 REgistry (SMACORE). Microorganisms [Internet]. MDPI AG; 2020 [cited 2020 Jul 11];8:695. Available from: https://www.mdpi.com/2076-2607/8/5/695.
Alzghari SK, Acuña VS. Supportive treatment with tocilizumab for COVID-19: a systematic review. J Clin Virol [Internet]. Elsevier B.V.; 2020 [cited 2020 Jul 11];127:104380. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7194791/.
Welcome — RECOVERY Trial [Internet]. [cited 2020 Jul 19]. Available from: https://www.recoverytrial.net/.
Brough HA, Kalayci O, Sediva A, Untersmayr E, Munblit D, Rodriguez P, et al. Managing childhood allergies and immunodeficiencies during respiratory virus epidemics – The 2020 COVID-19 pandemic : a statement from the EAACI-section on pediatrics. 2020;1–7.
Vultaggio A, Agache I, Akdis CA, Akdis M, Bavbek S, Bossios A, et al. Considerations on biologicals for patients with allergic disease in times of the COVID-19 pandemic: an EAACI Statement. Allergy [Internet]. Wiley; 2020 [cited 2020 Jul 17]; Available from: /pmc/articles/PMC7300800/?report=abstract.
Ogimi C, Englund JA, Bradford MC, Qin X, Boeckh M, Waghmare A. Characteristics and outcomes of Coronavirus Infection in Children: The role of viral factors and an immunocompromised state. J Pediatric Infect Dis Soc. 2019;8(1):21–28.
D’Antiga L. Coronaviruses and immunosuppressed patients: the facts during the third epidemic. Liver Transplant. 2020;26:832–4.
Fung M, Babik JM. COVID-19 in immunocompromised hosts: what we know so far [Internet]. Clin Infect Dis. 2020 Jun 27. [cited 2020 Jul 19]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7337668/.
Ciacchini B, Tonioli F, Marciano C, Faticato MG, Borali E, Pini Prato A, et al. Reluctance to seek pediatric care during the COVID-19 pandemic and the risks of delayed diagnosis. Ital J Pediatr [Internet]. BioMed Central; 2020 [cited 2020 Aug 27];46:87. Available from: https://doi.org/10.1186/s13052-020-00849-w.
Solis E, Hameed A, Brown K, Pleass H, Johnston E. Delayed emergency surgical presentation: impact of corona virus disease (COVID-19) on non-COVID patients [Internet]. ANZ J Surg. Blackwell Publishing; 2020 [cited 2020 Aug 29]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7280650/.
Pikoulis E, Solomos Z, Riza E, Puthoopparambil SJ, Pikoulis A, Karamagioli E, et al. Gathering evidence on the decreased emergency room visits during the coronavirus disease 19 pandemic [Internet]. Public Health. Elsevier B.V.; 2020 [cited 2020 Aug 29]. p. 42–3. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7247452/.
Saxena S, Skirrow H, Bedford H. Routine vaccination during covid-19 pandemic response [Internet]. BMJ. BMJ Publishing Group; 2020 [cited 2020 Aug 29]. Available from: https://doi.org/10.1136/bmj.m2392.
Shack AR, Arkush L, Reingold S, Weiser G. Masked paediatricians during the COVID -19 pandemic and communication with children. J Paediatr Child Health [Internet]. Blackwell Publishing; 2020 [cited 2020 Sep 5];jpc.15087. Available from: https://doi.org/10.1111/jpc.15087.
Education during COVID-19 and beyond A U G U S T 2 0 2 0.
The Lancet Child & Adolescent Health. Pandemic school closures: risks and opportunities [Internet]. Lancet Child Adolesc. Heal. Elsevier B.V.; 2020 [cited 2020 Aug 28]. p. 341. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7195509/.
Cortese S, Asherson P, Sonuga-Barke E, Banaschewski T, Brandeis D, Buitelaar J, et al. ADHD management during the COVID-19 pandemic: guidance from the European ADHD Guidelines Group [Internet]. Lancet Child Adolesc. Heal. Elsevier B.V.; 2020 [cited 2020 Jul 17]. p. 412–4. Available from: https://doi.org/10.1016/S2352-4642.
Zhang J, Shuai L, Yu H, Wang Z, Qiu M, Lu L, et al. Acute stress, behavioural symptoms and mood states among school-age children with attention-deficit/hyperactive disorder during the COVID-19 outbreak. Asian J Psychiatr [Internet]. Elsevier B.V.; 2020 [cited 2020 Jul 18];51:102077. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7195413/.
Cortese S, Coghill D, Santosh P, Hollis C, Simonoff E, ADHD Guidelines Group E. Starting ADHD medications during the COVID-19 pandemic: recommendations from the European ADHD Guidelines Group. Lancet child Adolesc Heal [Internet]. 2020 [cited 2020 Aug 28];4:e15. Available from.
Fegert JM, Vitiello B, Plener PL, Clemens V. Challenges and burden of the Coronavirus 2019 (COVID-19) pandemic for child and adolescent mental health: a narrative review to highlight clinical and research needs in the acute phase and the long return to normality [Internet]. Child Adolesc Psychiatry Ment Health. BioMed Central; 2020 [cited 2020 Aug 28]. p. 20. Available from: /pmc/articles/PMC7216870/?report=abstract.
Singh S, Roy D, Sinha K, Parveen S, Sharma G, Joshi G. Impact of COVID-19 and lockdown on mental health of children and adolescents: a narrative review with recommendations [Internet]. Psychiatry Res. Elsevier Ireland Ltd; 2020 [cited 2020 Sep 5]. Available from: https://pubmed.ncbi.nlm.nih.gov/32882598/.
Duan L, Shao X, Wang Y, Huang Y, Miao J, Yang X, et al. An investigation of mental health status of children and adolescents in china during the outbreak of COVID-19. J Affect Disord [Internet]. Elsevier B.V.; 2020 [cited 2020 Sep 5];275:112–8. Available from: https://pubmed.ncbi.nlm.nih.gov/32658812/.
Xie X, Xue Q, Zhou Y, Zhu K, Liu Q, Zhang J, et al. Mental health status among children in home confinement during the coronavirus disease 2019 outbreak in Hubei Province, China [Internet]. JAMA Pediatr. American Medical Association; 2020 [cited 2020 Jul 19]. Available from: /pmc/articles/PMC7182958/?report=abstract.
Teo S, Griffiths G. Child protection in the time of <scp>COVID</scp> -19. J Paediatr Child Health [Internet]. Blackwell Publishing; 2020 [cited 2020 Aug 28];56:838–40. Available from: https://doi.org/10.1111/jpc.14916.
Cluver L, Lachman JM, Sherr L, Wessels I, Krug E, Rakotomalala S, et al. Parenting in a time of COVID-19 [Internet]. Lancet. Lancet Publishing Group; 2020 [cited 2020 Aug 28]. p. e64. Available from: https://www.unicef.org/.
Silliman Cohen RI, Bosk EA. Vulnerable youth and the COVID-19 pandemic. Pediatrics [Internet]. American Academy of Pediatrics (AAP); 2020 [cited 2020 Jul 18];146:e20201306. Available from: https://doi.org/10.1542/peds.2020-1306.
Thomas EY, Anurudran A, Robb K, Burke TF. Spotlight on child abuse and neglect response in the time of COVID-19 [Internet]. Lancet Public Heal. Elsevier Ltd; 2020 [cited 2020 Aug 28]. p. e371. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7326432/.
Bryant DJ, Oo M, Damian AJ. The Rise of Adverse Childhood Experiences During the COVID-19 Pandemic. Psychol Trauma Theory, Res Pract Policy [Internet]. American Psychological Association Inc.; 2020 [cited 2020 Aug 28];12. Available from: https://pubmed.ncbi.nlm.nih.gov/32551773/.
Panthi B, Khanal P, Dahal M, Maharjan S, Nepal S. An urgent call to address the nutritional status of women and children in Nepal during COVID-19 crises [Internet]. Int. J. Equity Health. NLM (Medline); 2020 [cited 2020 Aug 27]. p. 87. Available from: https://doi.org/10.1186/s12939-020-01210-7.
Food Security and COVID-19 [Internet]. [cited 2020 Aug 28]. Available from: https://www.worldbank.org/en/topic/agriculture/brief/food-security-and-covid-19.
Brennan RJ, Nandy R. Complex humanitarian emergencies: a major global health challenge. Emerg Med Australas [Internet]. John Wiley & Sons, Ltd; 2001 [cited 2020 Aug 28];13:147–56. Available from: https://doi.org/10.1046/j.1442-2026.2001.00203.x.
Ruíz-Roso MB, de Carvalho Padilha P, Matilla-Escalante DC, Brun P, Ulloa N, Acevedo-Correa D, et al. Changes of physical activity and ultra-processed food consumption in adolescents from different countries during covid-19 pandemic: An observational study. Nutrients [Internet]. MDPI AG; 2020 [cited 2020 Aug 27];12:1–13. Available from: https://pubmed.ncbi.nlm.nih.gov/32751721/.
Pietrobelli A, Pecoraro L, Ferruzzi A, Heo M, Faith M, Zoller T, et al. Effects of COVID-19 lockdown on lifestyle behaviors in children with obesity living in Verona, Italy: a longitudinal study. Obesity [Internet]. Blackwell Publishing Inc.; 2020 [cited 2020 Aug 28];28:1382–5. Available from: https://pubmed.ncbi.nlm.nih.gov/32352652/.
Moore SA, Faulkner G, Rhodes RE, Brussoni M, Chulak-Bozzer T, Ferguson LJ, et al. Impact of the COVID-19 virus outbreak on movement and play behaviours of Canadian children and youth: a national survey. Int J Behav Nutr Phys Act [Internet]. BioMed Central; 2020 [cited 2020 Aug 28];17. Available from: https://pubmed.ncbi.nlm.nih.gov/32631350/.
Guan H, Okely AD, Aguilar-Farias N, del Pozo Cruz B, Draper CE, El Hamdouchi A, et al. Promoting healthy movement behaviours among children during the COVID-19 pandemic. 2020 [cited 2020 Aug 28]; Available from.
Hemphill NM, Kuan MTY, Harris KC. Reduced physical activity during COVID-19 pandemic in children with congenital heart disease. Can J Cardiol [Internet]. Elsevier Inc.; 2020 [cited 2020 Aug 28];36:1130–4. Available from: /pmc/articles/PMC7199682/?report=abstract.
Esposito S, Principi N. To mask or not to mask children to overcome COVID-19. Eur J Pediatr [Internet]. Springer; 2020 [cited 2020 Aug 29];179:1267–70. Available from: https://pubmed.ncbi.nlm.nih.gov/32388722/.
Draft landscape of COVID-19 candidate vaccines [Internet]. [cited 2020 Jul 26]. Available from: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines.
Jackson LA, Anderson EJ, Rouphael NG, Roberts PC, Makhene M, Coler RN, et al. An mRNA vaccine against SARS-CoV-2 - preliminary report. N Engl J Med [Internet]. N Engl J Med; 2020 [cited 2020 Jul 26]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/32663912.
Zhu F, Li Y, Guan X, Hou L, Wang W, Li J, et al. Safety , tolerability , and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine : Lancet [Internet]. Elsevier Ltd; 2020;395:1845–54. Available from: https://doi.org/10.1016/S0140-6736(20)31208-3.
Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S, Belij-rammerstorfer S, et al. Articles Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2 : a preliminary report of a phase 1 / 2 , single-blind , randomised controlled trial. :1–13.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Shira Rabinowicz, Itai M. Pessach and Eyal Leshem declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Tropical, Travel and Emerging Infections
Rights and permissions
About this article
Cite this article
Rabinowicz, S., Leshem, E. & Pessach, I.M. COVID-19 in the Pediatric Population—Review and Current Evidence. Curr Infect Dis Rep 22, 29 (2020). https://doi.org/10.1007/s11908-020-00739-6
Accepted:
Published:
DOI: https://doi.org/10.1007/s11908-020-00739-6