-
PDF
- Split View
-
Views
-
Cite
Cite
S Davis, RW Day, KJ Kopecky, MC Mahoney, PL McCarthy, AM Michalek, KB Moysich, LE Onstad, VF Stepanenko, PG Voillequé, T Chegerova, K Falkner, S Kulikov, E Maslova, V Ostapenko, N Rivkind, V Shevchuk, AF Tsyb, International Consortium For Research On The Health Effects Of Radiation. Writing Committee and Study Team:, Childhood leukaemia in Belarus, Russia, and Ukraine following the Chernobyl power station accident: results from an international collaborative population-based case–control study, International Journal of Epidemiology, Volume 35, Issue 2, April 2006, Pages 386–396, https://doi.org/10.1093/ije/dyi220
Close - Share Icon Share
Abstract
Background There is little evidence regarding the risk of leukaemia in children following exposure to radionuclides from the Chernobyl Nuclear Power Plant explosion on April 26, 1986.
Methods This population-based case–control study investigated whether acute leukaemia is increased among children who were in utero or <6 years of age at the time of the Chernobyl accident. Confirmed cases of leukaemia diagnosed from April 26, 1986 through December 31, 2000 in contaminated regions of Belarus, Russia, and Ukraine were included. Two controls were matched to each case on sex, birth year, and residence. Accumulated absorbed radiation dose to the bone marrow was estimated for each subject.
Results Median estimated radiation doses of participants were <10 mGy. A significant increase in leukaemia risk with increasing radiation dose to the bone marrow was found. This association was most evident in Ukraine, apparent (but not statistically significant) in Belarus, and not found in Russia.
Conclusion Taken at face value, these findings suggest that prolonged exposure to very low radiation doses may increase leukaemia risk as much as or even more than acute exposure. However the large and statistically significant dose–response might be accounted for, at least in part, by an overestimate of risk in Ukraine. Therefore, we conclude this study provides no convincing evidence of an increased risk of childhood leukaemia as a result of exposure to Chernobyl radiation, since it is unclear whether the results are due to a true radiation-related excess, asampling-derived bias in Ukraine, or some combination thereof. However, the lack of significant dose–responses in Belarus and Russia also cannot convincingly rule out the possibility of an increase in leukaemia risk at low dose levels.
Introduction
The explosion of Reactor Number 4 at the Chernobyl Nuclear Power Station on April 26, 1986 resulted in the largest accidental release of radionuclides into the environment in history. The radioactive plume contaminated large areas, and prevailing winds spread short-lived (primarily noble gases and radioiodines) and medium and long-lived (including 137Cs and 134Cs) radionuclides over portions of Ukraine, Belarus, Russia, areas of Europe, Scandinavia, and western Asia. The wide geographic dispersion of radionuclides, the large number of individuals potentially exposed, and concerns over the known leukaemogenic effect of ionizing radiation prompted the initiation of a number of epidemiological studies to investigate the role of Chernobyl-related radiation exposure and leukaemia risk.
To date, most of the studies have been descriptive.1 For example, the European Childhood Leukaemia-Lymphoma Incidence Study (ECLIS) examined trends in childhood (age 0–14 years) leukaemia based on cancer registration data from 23 countries; no significant associations were identified with exposure to radiation from Chernobyl.2–4 Other studies have examined changes in leukaemia incidence in the Former Soviet Union (FSU)5–9 and other European countries.10–16 Results from these studies do not provide consistent evidence linking childhood leukaemia rates in Europe with radiation exposures due to the Chernobyl accident,17 but are limited by dependence on historical and current registry data, the quality of which may have changed over time, regional differences in registry operations, and inaccuracy or unavailability of population data. In population studies based on registry data, differences in disease rates in areas characterized by high or low levels of contamination may be due to differences in population characteristics, or extensiveness of cancer registry coverage, rather than the effects of exposure. Only one case–control study18 has been published, which reports an ∼3-fold increase in the risk of leukaemia among persons who were 0–20 at the time of the accident (ATA) with highest radiation exposure levels compared with those with the lowest levels, in two regions (oblasts) of Ukraine (Rivno and Zhytomir) contaminated by Chernobyl fallout. Many of the youngest subjects in that study constituted a small subset of a larger ongoing study, the results of which we report here. This larger study was a multi-national population-based case–control study of acute leukaemia (AL) diagnosed among children who were <6 years of age ATA conducted in Belarus, Russia, and Ukraine. This study was designed to avoid the principal limitations of descriptive investigations by focusing on complete leukaemia case ascertainment and diagnostic verification in defined populations, and individual-level estimates of radiation dose and other exposures of potential aetiological significance.19
Methods
Case identification and control selection
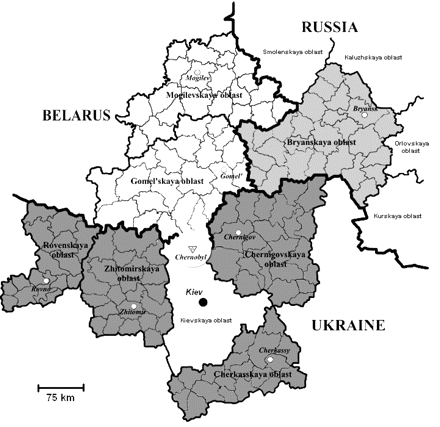
Details of the design of this study have been published elsewhere.19 Briefly, participants were recruited from areas of the FSU that were contaminated by radioactivity from the Chernobyl accident (Figure 1). Specifically, leukaemia cases and controls were identified from the Gomel'skaya and Mogilevskaya Oblasts in Belarus, the Bryanskaya Oblast in the Russian Federation (the most contaminated oblast in Russia), and the Rovenskaya, Zhytomirskaya, Chernigovskaya, and Cherkasskaya Oblasts in Ukraine (Chernigovskaya and Cherkasskaya Oblasts were on average significantly less contaminated than the Rovenskaya and Zhytomirskaya Oblasts). Cases of AL who were in utero or <6 years of age ATA and who were diagnosed in these geographic regions between April 26, 1986 and December 31, 2000, were identified from two types of sources, depending on the circumstances in each republic: records of regional cancer hospitals (oncodispensaries), and population-based tumour registries. In the Russian Federation cases were identified from the Bryansk Oncology Registry. In Belarus cases were identified from the national Belarus Tumor Registry and the Pediatric Oncology Center. In Ukraine cases were identified through manual searches of oncodispensary archival records. Once a potential case was identified, eligibility criteria (i.e. date of birth, date of diagnosis, and residence ATA) were ascertained through review of the medical record (in Russia, these were confirmed by personal interview).
Seven geographic regions (oblasts) in Belarus, Russia, and Ukraine in which the study population lived at the time of the Chernobyl accident. Small divisions within each oblast are raions. The central city or town of each oblast is indicated by a white circle and town name. The Chernobyl site is at the northern tip of the Kievskaya Oblast, which was not included in the study
The date of diagnosis of the case was defined as the reference date for purposes of selecting individually matched controls (who were required to be alive on the reference date) and delimiting the time period following the accident for which accumulated individual dose estimates were calculated. In all republics, controls were identified from the records of the polyclinics serving the population of each raion included in the study. All records were reviewed to identify potential controls that could be matched to a case on sex, birth year, and oblast of residence ATA. However, the protocol for further matching by raion of residence differed between republics (a raion is roughly equivalent to a county in the US). In Russia, controls were matched to cases on raion of residence of the case ATA. In Ukraine, controls were initially matched on raion of residence of the case ATA (118 or 22% of the controls), and subsequently were to be randomly selected from a raion different from that of the case, but within the same oblast. In Belarus, controls were matched to cases on raion of residence on the reference date and randomly selected from any raion of residence ATA with the selection weighted by raion population. A total of 20 potential controls were selected from the polyclinic records for each case and interviews were scheduled for the first two. If the first or second potential controls could not be reached, the next individuals on the list were contacted and scheduled for an interview. This process was continued until two controls were enrolled in the study for each case.
Personal interview
An in-person interview, administered by trained physician and dosimetry interviewers, was conducted for each case and control. Since many of the cases were deceased, interviews were conducted with someone with personal knowledge of the subject's life up to the reference date, preferably the subject's mother or another close relative. The interview schedule was developed by both FSU and US scientists and included a demographic questionnaire, general health interview, leukaemia risk factor questionnaire, a brief maternal and paternal occupational history, and a detailed dosimetry questionnaire. The latter instrument included questions related to the sources and consumption of foods and milk; residence and occupation; relocation and migration; personal protective measures after the accident; type of house; and time spent indoors and outdoors after the accident. All procedures and data collection instruments were approved by Institutional Review Boards at the US institutions participating in this research and in Belarus, the Russian Federation, and Ukraine. All study participants provided written informed consent to participate prior to data collection.
Confirmation of leukaemia diagnoses
A Leukaemia Diagnostic Working Group (LDWG) consisting of haematologic morphologists and haematologists from Belarus, Israel, Russia, Ukraine, and the United States was created to verify that all cases included for study were indeed AL. Of 463 identified potential cases, a confirmed diagnosis of AL was made in 442 (95%). The potential cases included 77 with no adequate slides available for review, of whom 67 were confirmed as AL and included in this study based on review of clinical information that included the results of laboratory tests, the name of the institution where the diagnosis was made, and whether the patient received therapy. Table 1 summarizes the LDWG consensus diagnosis of the 463 potential cases. The 21 potential cases who were excluded were determined by the LDWG to have the following diagnoses: myelodysplastic syndrome (n = 4), chronic myelogenous leukaemia (n = 4), other diagnoses (n = 3), and no determination (n = 10 cases without slides available for review and insufficient clinical information to confirm the diagnosis of AL). Of the 442 confirmed cases, 21 were excluded owing to ineligibility: age >6 years ATA or residence outside of the seven study oblasts ATA. Among the 421 cases included in the study were 311 (74%) with acute lymphoid leukaemia (ALL) and 86 with acute myeloid leukaemia (AML) (21%). This distribution of leukaemia subtype is generally consistent with what would be expected based on general population estimates, given the age restriction for inclusion in the present study. A more detailed description of the LDWG results is published elsewhere.20
Leukaemia Diagnostic Working Group (LDWG) review diagnoses of 463 potential and 421 included cases
| . | Belarus . | . | Russia . | . | Ukraine . | . | Combined . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LDWG diagnosis . | n . | % . | n . | % . | n . | % . | n . | % . | ||||||||
| Potential cases reviewed by LDWG | ||||||||||||||||
| ALLa | 90 | 71 | 30 | 61 | 203 | 71 | 323 | 70 | ||||||||
| AMLb | 20 | 16 | 9 | 18 | 61 | 21 | 90 | 19 | ||||||||
| ALUc | 11 | 9 | 0 | 0 | 18 | 6 | 29 | 6 | ||||||||
| Total AL | 121 | 96 | 39 | 79 | 282 | 98 | 442 | 95 | ||||||||
| CMLd | 0 | 0 | 0 | 0 | 4 | 1 | 4 | 1 | ||||||||
| MDSe | 1 | 1 | 3 | 6 | 0 | 0 | 4 | 1 | ||||||||
| Other | 2 | 2 | 0 | 0 | 1 | <1 | 3 | 1 | ||||||||
| Inadequate materials | 3 | 2 | 7 | 14 | 0 | 0 | 10 | 2 | ||||||||
| Total | 127 | 100 | 49 | 100 | 287 | 100 | 463 | 100 | ||||||||
| Included cases | ||||||||||||||||
| ALLa | 84 | 74 | 30 | 77 | 197 | 74 | 311 | 74 | ||||||||
| AMLb | 20 | 18 | 9 | 23 | 57 | 21 | 86 | 21 | ||||||||
| ALUc | 10 | 9 | 0 | 0 | 14 | 5 | 24 | 5 | ||||||||
| Total | 114 | 100 | 39 | 100 | 268 | 100 | 421 | 100 | ||||||||
| . | Belarus . | . | Russia . | . | Ukraine . | . | Combined . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LDWG diagnosis . | n . | % . | n . | % . | n . | % . | n . | % . | ||||||||
| Potential cases reviewed by LDWG | ||||||||||||||||
| ALLa | 90 | 71 | 30 | 61 | 203 | 71 | 323 | 70 | ||||||||
| AMLb | 20 | 16 | 9 | 18 | 61 | 21 | 90 | 19 | ||||||||
| ALUc | 11 | 9 | 0 | 0 | 18 | 6 | 29 | 6 | ||||||||
| Total AL | 121 | 96 | 39 | 79 | 282 | 98 | 442 | 95 | ||||||||
| CMLd | 0 | 0 | 0 | 0 | 4 | 1 | 4 | 1 | ||||||||
| MDSe | 1 | 1 | 3 | 6 | 0 | 0 | 4 | 1 | ||||||||
| Other | 2 | 2 | 0 | 0 | 1 | <1 | 3 | 1 | ||||||||
| Inadequate materials | 3 | 2 | 7 | 14 | 0 | 0 | 10 | 2 | ||||||||
| Total | 127 | 100 | 49 | 100 | 287 | 100 | 463 | 100 | ||||||||
| Included cases | ||||||||||||||||
| ALLa | 84 | 74 | 30 | 77 | 197 | 74 | 311 | 74 | ||||||||
| AMLb | 20 | 18 | 9 | 23 | 57 | 21 | 86 | 21 | ||||||||
| ALUc | 10 | 9 | 0 | 0 | 14 | 5 | 24 | 5 | ||||||||
| Total | 114 | 100 | 39 | 100 | 268 | 100 | 421 | 100 | ||||||||
Acute lymphoid leukaemia.
Acute myeloid leukaemia.
AL, unclassified.
Chronic myelogenous leukaemia.
Myelodysplastic syndrome.
Leukaemia Diagnostic Working Group (LDWG) review diagnoses of 463 potential and 421 included cases
| . | Belarus . | . | Russia . | . | Ukraine . | . | Combined . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LDWG diagnosis . | n . | % . | n . | % . | n . | % . | n . | % . | ||||||||
| Potential cases reviewed by LDWG | ||||||||||||||||
| ALLa | 90 | 71 | 30 | 61 | 203 | 71 | 323 | 70 | ||||||||
| AMLb | 20 | 16 | 9 | 18 | 61 | 21 | 90 | 19 | ||||||||
| ALUc | 11 | 9 | 0 | 0 | 18 | 6 | 29 | 6 | ||||||||
| Total AL | 121 | 96 | 39 | 79 | 282 | 98 | 442 | 95 | ||||||||
| CMLd | 0 | 0 | 0 | 0 | 4 | 1 | 4 | 1 | ||||||||
| MDSe | 1 | 1 | 3 | 6 | 0 | 0 | 4 | 1 | ||||||||
| Other | 2 | 2 | 0 | 0 | 1 | <1 | 3 | 1 | ||||||||
| Inadequate materials | 3 | 2 | 7 | 14 | 0 | 0 | 10 | 2 | ||||||||
| Total | 127 | 100 | 49 | 100 | 287 | 100 | 463 | 100 | ||||||||
| Included cases | ||||||||||||||||
| ALLa | 84 | 74 | 30 | 77 | 197 | 74 | 311 | 74 | ||||||||
| AMLb | 20 | 18 | 9 | 23 | 57 | 21 | 86 | 21 | ||||||||
| ALUc | 10 | 9 | 0 | 0 | 14 | 5 | 24 | 5 | ||||||||
| Total | 114 | 100 | 39 | 100 | 268 | 100 | 421 | 100 | ||||||||
| . | Belarus . | . | Russia . | . | Ukraine . | . | Combined . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LDWG diagnosis . | n . | % . | n . | % . | n . | % . | n . | % . | ||||||||
| Potential cases reviewed by LDWG | ||||||||||||||||
| ALLa | 90 | 71 | 30 | 61 | 203 | 71 | 323 | 70 | ||||||||
| AMLb | 20 | 16 | 9 | 18 | 61 | 21 | 90 | 19 | ||||||||
| ALUc | 11 | 9 | 0 | 0 | 18 | 6 | 29 | 6 | ||||||||
| Total AL | 121 | 96 | 39 | 79 | 282 | 98 | 442 | 95 | ||||||||
| CMLd | 0 | 0 | 0 | 0 | 4 | 1 | 4 | 1 | ||||||||
| MDSe | 1 | 1 | 3 | 6 | 0 | 0 | 4 | 1 | ||||||||
| Other | 2 | 2 | 0 | 0 | 1 | <1 | 3 | 1 | ||||||||
| Inadequate materials | 3 | 2 | 7 | 14 | 0 | 0 | 10 | 2 | ||||||||
| Total | 127 | 100 | 49 | 100 | 287 | 100 | 463 | 100 | ||||||||
| Included cases | ||||||||||||||||
| ALLa | 84 | 74 | 30 | 77 | 197 | 74 | 311 | 74 | ||||||||
| AMLb | 20 | 18 | 9 | 23 | 57 | 21 | 86 | 21 | ||||||||
| ALUc | 10 | 9 | 0 | 0 | 14 | 5 | 24 | 5 | ||||||||
| Total | 114 | 100 | 39 | 100 | 268 | 100 | 421 | 100 | ||||||||
Acute lymphoid leukaemia.
Acute myeloid leukaemia.
AL, unclassified.
Chronic myelogenous leukaemia.
Myelodysplastic syndrome.
Dosimetry
Accumulated absorbed radiation dose to the bone marrow and corresponding uncertainties were estimated for each case and matched control from the time of the accident until the reference date. The Dosimetry Working Group comprised of investigators from Belarus, Russia, Ukraine, and the United States developed a common methodology for calculating individualized dose estimates from external radiation fields and from ingestion of contaminated foods. The dosimetry methods reflect changes in external exposure rates and levels of food contamination with time following the accident, as well as local conditions.
The dose estimation methods used individual dosimetry parameters that reflected local conditions at each location where each subject lived during the appropriate time interval. The dosimetry questionnaire described above provided residence history and other pertinent personal information. For study subjects born after April 26, 1986, the mother's residence and dietary history were also obtained in order to estimate the dose received while the subject was in utero (61 cases). Consumption of mother's milk by the child through breastfeeding is included when calculating internal doses.
Calculations of external dose for a particular residence location employed data derived from field measurements in the three republics as well as information about individual subjects obtained from the questionnaires. In the first category were data on concentrations of radionuclides in soils, time-dependent exposure rates, shielding factors, and date and times of radioactive cloud arrivals. Soil contamination levels were characterized by the 137Cs deposition density and the ratios of contamination levels relative to 137Cs for other radionuclides separately for each of the raions in the seven oblasts. For each subject, the questionnaire yielded a residence history from the time of the accident to the reference date, type of dwelling at each location, and percentages of time spent outdoors during that period.
The internal dose estimates also relied upon the soil contamination levels at the residence locations. Responses to the questionnaire provided information about individual milk consumption, milk sources and types, and the sources and consumption of other foods. Information based on environmental measurements included raion average soil-to-milk transfer coefficients for radiocaesium (or soil types to estimate such transfer), ratios of 137Cs concentrations measured in various foods to those measured in milk (which changed with time after the accident), the contribution of 134Cs, and losses of radioactivity during food preparation and cooking.
The uncertainties of individual doses were estimated by Monte Carlo simulation of the variability of each of the input parameters used for dose calculations. In order to estimate the uncertainties of individual doses, 1000 realizations of dose calculations were performed on each person using the stochastic variation (distributions) of the input parameters.
Data management and quality assurance
The research team in each country entered the demographic, dosimetric, and other data into a study-specific database designed by the Data Coordination Office (DCO) in Moscow. Data audits were conducted in all three republics to assure the implementation of uniform study methodology across the three study sites (variation in control selection is the exception). Audit procedures included: (i) an assessment of study methodology, involving independent verbal accounts of the research procedures by project managers, interviewers, and data managers; (ii) monitoring interview procedures by conducting duplicate interviews using an abbreviated version of the interview schedule, and (iii) monitoring data entry accuracy by randomly selecting paper records and determining discrepancies between information from paper records and the electronic database. These data were reviewed for quality, consistency, and completeness. Only after all these quality control checks were completed did each team submit its data to the DCO for assembly into a combined file for statistical analyses.
Statistical methods
Results
Characteristics of cases and controls
Of the 421 confirmed cases of AL included in the analysis, 44% (n = 185) were female, the median age ATA (age at the time of the accident) was 2.3 years, and 14% (n = 61) received at least some in utero exposure (Table 2). Approximately 76% (n = 319) were born during 1981–85 and nearly half (52%; n = 219) of the cases were diagnosed before 1991. The median age at diagnosis was ∼7 years, and 8% (n = 35) were diagnosed before 3 years of age. Approximately half of the cases were diagnosed between the ages of 5 and 10. This primarily reflects the restriction of age ATA as an inclusion criterion and is different from what would be expected in an unrestricted sample from a steady-state population. There were no significant differences by country regarding the distribution of sex, year of diagnosis, or age at diagnosis. There was some evidence of heterogeneity of age ATA between the countries (P = 0.02). However, there was no evidence that the cases from any country tended to be consistently older or younger ATA (P = 0.35).
Characteristics of 421 included leukaemia cases
| . | Belarus (n = 114) . | . | Russia (n = 39) . | . | Ukraine (n = 268) . | . | Combined (n = 421) . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic . | n . | % . | n . | % . | n . | % . | n . | % . | P-valuea . | ||||
| Sex | 0.78 | ||||||||||||
| Female | 47 | 41 | 18 | 46 | 120 | 45 | 185 | 44 | |||||
| Male | 67 | 59 | 21 | 54 | 148 | 55 | 236 | 56 | |||||
| Age (years) on April 26, 1986 (Age ATA) | 0.02 | ||||||||||||
| In utero | 14 | 12 | 3 | 8 | 44 | 16 | 61 | 14 | |||||
| <1 | 22 | 19 | 8 | 21 | 41 | 15 | 71 | 17 | |||||
| 1 | 22 | 19 | 4 | 10 | 30 | 11 | 56 | 13 | |||||
| 2 | 17 | 15 | 11 | 28 | 47 | 18 | 75 | 18 | |||||
| 3 | 7 | 6 | 9 | 23 | 43 | 16 | 59 | 14 | |||||
| 4 | 20 | 18 | 4 | 10 | 42 | 16 | 66 | 16 | |||||
| 5 | 12 | 11 | 0 | 0 | 21 | 8 | 33 | 8 | |||||
| Median | 2.0 | 2.4 | 2.4 | 2.3 | 0.35 | ||||||||
| Minimum–maximum | −0.8–6.0 | −0.3–4.9 | −0.8–6.0 | −0.8–6.0 | |||||||||
| Year of diagnosis | 0.53 | ||||||||||||
| 1986–90 | 61 | 54 | 16 | 41 | 142 | 53 | 219 | 52 | |||||
| 1991–95 | 34 | 30 | 13 | 33 | 84 | 31 | 131 | 31 | |||||
| 1996–2000 | 19 | 17 | 10 | 26 | 42 | 16 | 71 | 17 | |||||
| Age (years) at diagnosis | 0.40 | ||||||||||||
| <1 | 0 | 0 | 0 | 0 | 3 | 1 | 3 | 1 | |||||
| 1–2 | 10 | 9 | 1 | 3 | 21 | 8 | 32 | 8 | |||||
| 3–4 | 21 | 18 | 10 | 26 | 72 | 27 | 103 | 24 | |||||
| 5–10 | 58 | 51 | 18 | 46 | 110 | 41 | 186 | 44 | |||||
| 11–15 | 21 | 18 | 9 | 23 | 59 | 22 | 89 | 21 | |||||
| 16–19 | 4 | 4 | 1 | 3 | 3 | 1 | 8 | 2 | |||||
| Median | 6.6 | 8.1 | 6.9 | 6.9 | 0.25 | ||||||||
| Minimum–maximum | 1.3–18.7 | 2.6–18.2 | 0–17.1 | 0–18.7 | |||||||||
| . | Belarus (n = 114) . | . | Russia (n = 39) . | . | Ukraine (n = 268) . | . | Combined (n = 421) . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic . | n . | % . | n . | % . | n . | % . | n . | % . | P-valuea . | ||||
| Sex | 0.78 | ||||||||||||
| Female | 47 | 41 | 18 | 46 | 120 | 45 | 185 | 44 | |||||
| Male | 67 | 59 | 21 | 54 | 148 | 55 | 236 | 56 | |||||
| Age (years) on April 26, 1986 (Age ATA) | 0.02 | ||||||||||||
| In utero | 14 | 12 | 3 | 8 | 44 | 16 | 61 | 14 | |||||
| <1 | 22 | 19 | 8 | 21 | 41 | 15 | 71 | 17 | |||||
| 1 | 22 | 19 | 4 | 10 | 30 | 11 | 56 | 13 | |||||
| 2 | 17 | 15 | 11 | 28 | 47 | 18 | 75 | 18 | |||||
| 3 | 7 | 6 | 9 | 23 | 43 | 16 | 59 | 14 | |||||
| 4 | 20 | 18 | 4 | 10 | 42 | 16 | 66 | 16 | |||||
| 5 | 12 | 11 | 0 | 0 | 21 | 8 | 33 | 8 | |||||
| Median | 2.0 | 2.4 | 2.4 | 2.3 | 0.35 | ||||||||
| Minimum–maximum | −0.8–6.0 | −0.3–4.9 | −0.8–6.0 | −0.8–6.0 | |||||||||
| Year of diagnosis | 0.53 | ||||||||||||
| 1986–90 | 61 | 54 | 16 | 41 | 142 | 53 | 219 | 52 | |||||
| 1991–95 | 34 | 30 | 13 | 33 | 84 | 31 | 131 | 31 | |||||
| 1996–2000 | 19 | 17 | 10 | 26 | 42 | 16 | 71 | 17 | |||||
| Age (years) at diagnosis | 0.40 | ||||||||||||
| <1 | 0 | 0 | 0 | 0 | 3 | 1 | 3 | 1 | |||||
| 1–2 | 10 | 9 | 1 | 3 | 21 | 8 | 32 | 8 | |||||
| 3–4 | 21 | 18 | 10 | 26 | 72 | 27 | 103 | 24 | |||||
| 5–10 | 58 | 51 | 18 | 46 | 110 | 41 | 186 | 44 | |||||
| 11–15 | 21 | 18 | 9 | 23 | 59 | 22 | 89 | 21 | |||||
| 16–19 | 4 | 4 | 1 | 3 | 3 | 1 | 8 | 2 | |||||
| Median | 6.6 | 8.1 | 6.9 | 6.9 | 0.25 | ||||||||
| Minimum–maximum | 1.3–18.7 | 2.6–18.2 | 0–17.1 | 0–18.7 | |||||||||
Based on the Pearson's chi-square test for differences in proportions and the median test for differences in location.
Characteristics of 421 included leukaemia cases
| . | Belarus (n = 114) . | . | Russia (n = 39) . | . | Ukraine (n = 268) . | . | Combined (n = 421) . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic . | n . | % . | n . | % . | n . | % . | n . | % . | P-valuea . | ||||
| Sex | 0.78 | ||||||||||||
| Female | 47 | 41 | 18 | 46 | 120 | 45 | 185 | 44 | |||||
| Male | 67 | 59 | 21 | 54 | 148 | 55 | 236 | 56 | |||||
| Age (years) on April 26, 1986 (Age ATA) | 0.02 | ||||||||||||
| In utero | 14 | 12 | 3 | 8 | 44 | 16 | 61 | 14 | |||||
| <1 | 22 | 19 | 8 | 21 | 41 | 15 | 71 | 17 | |||||
| 1 | 22 | 19 | 4 | 10 | 30 | 11 | 56 | 13 | |||||
| 2 | 17 | 15 | 11 | 28 | 47 | 18 | 75 | 18 | |||||
| 3 | 7 | 6 | 9 | 23 | 43 | 16 | 59 | 14 | |||||
| 4 | 20 | 18 | 4 | 10 | 42 | 16 | 66 | 16 | |||||
| 5 | 12 | 11 | 0 | 0 | 21 | 8 | 33 | 8 | |||||
| Median | 2.0 | 2.4 | 2.4 | 2.3 | 0.35 | ||||||||
| Minimum–maximum | −0.8–6.0 | −0.3–4.9 | −0.8–6.0 | −0.8–6.0 | |||||||||
| Year of diagnosis | 0.53 | ||||||||||||
| 1986–90 | 61 | 54 | 16 | 41 | 142 | 53 | 219 | 52 | |||||
| 1991–95 | 34 | 30 | 13 | 33 | 84 | 31 | 131 | 31 | |||||
| 1996–2000 | 19 | 17 | 10 | 26 | 42 | 16 | 71 | 17 | |||||
| Age (years) at diagnosis | 0.40 | ||||||||||||
| <1 | 0 | 0 | 0 | 0 | 3 | 1 | 3 | 1 | |||||
| 1–2 | 10 | 9 | 1 | 3 | 21 | 8 | 32 | 8 | |||||
| 3–4 | 21 | 18 | 10 | 26 | 72 | 27 | 103 | 24 | |||||
| 5–10 | 58 | 51 | 18 | 46 | 110 | 41 | 186 | 44 | |||||
| 11–15 | 21 | 18 | 9 | 23 | 59 | 22 | 89 | 21 | |||||
| 16–19 | 4 | 4 | 1 | 3 | 3 | 1 | 8 | 2 | |||||
| Median | 6.6 | 8.1 | 6.9 | 6.9 | 0.25 | ||||||||
| Minimum–maximum | 1.3–18.7 | 2.6–18.2 | 0–17.1 | 0–18.7 | |||||||||
| . | Belarus (n = 114) . | . | Russia (n = 39) . | . | Ukraine (n = 268) . | . | Combined (n = 421) . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic . | n . | % . | n . | % . | n . | % . | n . | % . | P-valuea . | ||||
| Sex | 0.78 | ||||||||||||
| Female | 47 | 41 | 18 | 46 | 120 | 45 | 185 | 44 | |||||
| Male | 67 | 59 | 21 | 54 | 148 | 55 | 236 | 56 | |||||
| Age (years) on April 26, 1986 (Age ATA) | 0.02 | ||||||||||||
| In utero | 14 | 12 | 3 | 8 | 44 | 16 | 61 | 14 | |||||
| <1 | 22 | 19 | 8 | 21 | 41 | 15 | 71 | 17 | |||||
| 1 | 22 | 19 | 4 | 10 | 30 | 11 | 56 | 13 | |||||
| 2 | 17 | 15 | 11 | 28 | 47 | 18 | 75 | 18 | |||||
| 3 | 7 | 6 | 9 | 23 | 43 | 16 | 59 | 14 | |||||
| 4 | 20 | 18 | 4 | 10 | 42 | 16 | 66 | 16 | |||||
| 5 | 12 | 11 | 0 | 0 | 21 | 8 | 33 | 8 | |||||
| Median | 2.0 | 2.4 | 2.4 | 2.3 | 0.35 | ||||||||
| Minimum–maximum | −0.8–6.0 | −0.3–4.9 | −0.8–6.0 | −0.8–6.0 | |||||||||
| Year of diagnosis | 0.53 | ||||||||||||
| 1986–90 | 61 | 54 | 16 | 41 | 142 | 53 | 219 | 52 | |||||
| 1991–95 | 34 | 30 | 13 | 33 | 84 | 31 | 131 | 31 | |||||
| 1996–2000 | 19 | 17 | 10 | 26 | 42 | 16 | 71 | 17 | |||||
| Age (years) at diagnosis | 0.40 | ||||||||||||
| <1 | 0 | 0 | 0 | 0 | 3 | 1 | 3 | 1 | |||||
| 1–2 | 10 | 9 | 1 | 3 | 21 | 8 | 32 | 8 | |||||
| 3–4 | 21 | 18 | 10 | 26 | 72 | 27 | 103 | 24 | |||||
| 5–10 | 58 | 51 | 18 | 46 | 110 | 41 | 186 | 44 | |||||
| 11–15 | 21 | 18 | 9 | 23 | 59 | 22 | 89 | 21 | |||||
| 16–19 | 4 | 4 | 1 | 3 | 3 | 1 | 8 | 2 | |||||
| Median | 6.6 | 8.1 | 6.9 | 6.9 | 0.25 | ||||||||
| Minimum–maximum | 1.3–18.7 | 2.6–18.2 | 0–17.1 | 0–18.7 | |||||||||
Based on the Pearson's chi-square test for differences in proportions and the median test for differences in location.
Among the 842 controls matched to the 421 included cases, seven were later found to be ineligible and, therefore, excluded from analysis. Interviews were most commonly conducted with the subject's mother (90% of cases, 91% of controls) or father (5% of both cases and controls). Parents' level of education, an approximate indicator of socioeconomic status, was generally similar between cases and controls, although parents of controls were somewhat more likely to have received college or university education (14% for both mothers and fathers) than parents of cases (9% for both).
Diagnostic medical radiation exposures to the head and neck were reported for only four cases (1%) and seven controls (1%), and histories of dental X-ray exposure were reported for only one case (<1%) and five controls (1%). Much more common were histories of chest fluoroscopy, reported for 66 cases (16%) and 71 controls (9%), and other external diagnostic radiation procedures, reported for 57 cases (14%) and 79 controls (9%). More than half of the cases with histories of chest fluoroscopy had such an examination in the calendar year of the reference date or the preceding calendar year. The same was true of controls. For other external diagnostic radiation exposures, more than half of subjects with such examinations had them in the calendar year of the reference date or in the preceding 2 years (cases) or 3 years (controls). The higher frequency of such histories among cases might result at least partly from increased medical attention to cases before their diagnoses of AL. One case (<1%) and three controls (<1%) had histories of internal (oral or injected) diagnostic radiation exposures. Only four cases (1%) and two controls (<1%) had any prior history of cancer or other serious diseases, and only one participant, a case, had a history of prior radiation therapy for cancer or any other condition.
Analyses of estimated doses and dose–response
Table 3 summarizes the distribution of total estimated radiation dose to the bone marrow calculated using the common methodology, for each country separately and for all countries combined. Overall, the mean dose was higher among cases (10.8 mGy) than controls (6.3 mGy). Mean doses were similar in Belarus and Russia, and there was little difference between cases and controls in these two countries. In contrast, the mean dose for cases in Ukraine was substantially higher (10.1 mGy) than for controls (3.5 mGy), and was somewhat lower than for cases and controls in Belarus and for controls in Russia. There was less difference in median doses between cases (0.9 mGy) and controls (0.7 mGy), and median doses were considerably lower than the means, reflecting a highly skewed distribution of doses. Median doses were lowest in Ukraine (0.5 mGy for cases and 0.4 mGy for controls) and highest in Belarus (5.6 mGy for cases and 5.0 mGy for controls). It is also evident in the bottom section of Table 3 that a substantially lower proportion of cases, and particularly controls, were in the highest dose category (≥5 mGy) in Ukraine compared with the other two countries.
Estimated radiation doses, by republic and case–control status
| . | Belarus . | . | Russia . | . | Ukraine . | . | Combined . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimated total . | Case . | Control . | Case . | Control . | Case . | Control . | Case . | Control . | ||||
| dose (mGy) . | (n = 114) . | (n = 221) . | (n = 39) . | (n = 78) . | (n = 268) . | (n = 536) . | (n = 421) . | (n = 835) . | ||||
| Minimum | 0.10 | 0.04 | 0.40 | 0.19 | 0.00 | 0.00 | 0.00 | 0.00 | ||||
| Maximum | 144.88 | 186.19 | 88.89 | 202.38 | 390.58 | 265.33 | 390.58 | 265.33 | ||||
| Median | 5.61 | 5.02 | 1.38 | 1.09 | 0.50 | 0.39 | 0.93 | 0.65 | ||||
| Mean | 12.81 | 11.74 | 9.97 | 10.49 | 10.12 | 3.46 | 10.84 | 6.30 | ||||
| . | Belarus . | . | Russia . | . | Ukraine . | . | Combined . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimated total . | Case . | Control . | Case . | Control . | Case . | Control . | Case . | Control . | ||||
| dose (mGy) . | (n = 114) . | (n = 221) . | (n = 39) . | (n = 78) . | (n = 268) . | (n = 536) . | (n = 421) . | (n = 835) . | ||||
| Minimum | 0.10 | 0.04 | 0.40 | 0.19 | 0.00 | 0.00 | 0.00 | 0.00 | ||||
| Maximum | 144.88 | 186.19 | 88.89 | 202.38 | 390.58 | 265.33 | 390.58 | 265.33 | ||||
| Median | 5.61 | 5.02 | 1.38 | 1.09 | 0.50 | 0.39 | 0.93 | 0.65 | ||||
| Mean | 12.81 | 11.74 | 9.97 | 10.49 | 10.12 | 3.46 | 10.84 | 6.30 | ||||
. | n . | % . | n . | % . | n . | % . | n . | % . | n . | % . | n . | % . | n . | % . | n . | % . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <1.0 | 26 | 23 | 57 | 26 | 17 | 44 | 34 | 44 | 176 | 66 | 409 | 76 | 219 | 52 | 500 | 60 |
| 1.0–4.999 | 26 | 23 | 53 | 24 | 10 | 26 | 25 | 32 | 47 | 18 | 85 | 16 | 83 | 20 | 163 | 20 |
| ≥5.0 | 62 | 54 | 111 | 50 | 12 | 31 | 19 | 24 | 45 | 17 | 42 | 8 | 119 | 28 | 172 | 21 |
. | n . | % . | n . | % . | n . | % . | n . | % . | n . | % . | n . | % . | n . | % . | n . | % . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <1.0 | 26 | 23 | 57 | 26 | 17 | 44 | 34 | 44 | 176 | 66 | 409 | 76 | 219 | 52 | 500 | 60 |
| 1.0–4.999 | 26 | 23 | 53 | 24 | 10 | 26 | 25 | 32 | 47 | 18 | 85 | 16 | 83 | 20 | 163 | 20 |
| ≥5.0 | 62 | 54 | 111 | 50 | 12 | 31 | 19 | 24 | 45 | 17 | 42 | 8 | 119 | 28 | 172 | 21 |
Estimated radiation doses, by republic and case–control status
| . | Belarus . | . | Russia . | . | Ukraine . | . | Combined . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimated total . | Case . | Control . | Case . | Control . | Case . | Control . | Case . | Control . | ||||
| dose (mGy) . | (n = 114) . | (n = 221) . | (n = 39) . | (n = 78) . | (n = 268) . | (n = 536) . | (n = 421) . | (n = 835) . | ||||
| Minimum | 0.10 | 0.04 | 0.40 | 0.19 | 0.00 | 0.00 | 0.00 | 0.00 | ||||
| Maximum | 144.88 | 186.19 | 88.89 | 202.38 | 390.58 | 265.33 | 390.58 | 265.33 | ||||
| Median | 5.61 | 5.02 | 1.38 | 1.09 | 0.50 | 0.39 | 0.93 | 0.65 | ||||
| Mean | 12.81 | 11.74 | 9.97 | 10.49 | 10.12 | 3.46 | 10.84 | 6.30 | ||||
| . | Belarus . | . | Russia . | . | Ukraine . | . | Combined . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimated total . | Case . | Control . | Case . | Control . | Case . | Control . | Case . | Control . | ||||
| dose (mGy) . | (n = 114) . | (n = 221) . | (n = 39) . | (n = 78) . | (n = 268) . | (n = 536) . | (n = 421) . | (n = 835) . | ||||
| Minimum | 0.10 | 0.04 | 0.40 | 0.19 | 0.00 | 0.00 | 0.00 | 0.00 | ||||
| Maximum | 144.88 | 186.19 | 88.89 | 202.38 | 390.58 | 265.33 | 390.58 | 265.33 | ||||
| Median | 5.61 | 5.02 | 1.38 | 1.09 | 0.50 | 0.39 | 0.93 | 0.65 | ||||
| Mean | 12.81 | 11.74 | 9.97 | 10.49 | 10.12 | 3.46 | 10.84 | 6.30 | ||||
. | n . | % . | n . | % . | n . | % . | n . | % . | n . | % . | n . | % . | n . | % . | n . | % . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <1.0 | 26 | 23 | 57 | 26 | 17 | 44 | 34 | 44 | 176 | 66 | 409 | 76 | 219 | 52 | 500 | 60 |
| 1.0–4.999 | 26 | 23 | 53 | 24 | 10 | 26 | 25 | 32 | 47 | 18 | 85 | 16 | 83 | 20 | 163 | 20 |
| ≥5.0 | 62 | 54 | 111 | 50 | 12 | 31 | 19 | 24 | 45 | 17 | 42 | 8 | 119 | 28 | 172 | 21 |
. | n . | % . | n . | % . | n . | % . | n . | % . | n . | % . | n . | % . | n . | % . | n . | % . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <1.0 | 26 | 23 | 57 | 26 | 17 | 44 | 34 | 44 | 176 | 66 | 409 | 76 | 219 | 52 | 500 | 60 |
| 1.0–4.999 | 26 | 23 | 53 | 24 | 10 | 26 | 25 | 32 | 47 | 18 | 85 | 16 | 83 | 20 | 163 | 20 |
| ≥5.0 | 62 | 54 | 111 | 50 | 12 | 31 | 19 | 24 | 45 | 17 | 42 | 8 | 119 | 28 | 172 | 21 |
Analyses of the radiation dose–response are summarized in Table 4. Using <1.0 mGy as the baseline category, the odds ratio for AL was estimated as 1.46 and 2.60 for doses of 1.0–4.999 and ≥5 mGy, respectively, for all republics combined. In Ukraine the odds ratio in the highest dose category was 3.50 with 95% CI that excludes the value of 1.0 (1.995–6.15). The odds ratios for ≥5 mGy were also >1.0 for both Belarus and Russia, although the corresponding CIs included 1.0. Based on the loglinear model for the odds ratio, leukaemia risk increased significantly with increasing radiation dose for all republics combined (one-tailed P-value = 0.0030). This is largely accounted for by the significant dose–response in Ukraine (P = 0.005). Although the heterogeneity of dose–response among the three republics was not statistically significant (P = 0.26), the estimated regression coefficient for Ukraine was roughly five times greater than the estimate for Belarus. The dose–response was not statistically significant in either Belarus (P = 0.33) or Russia (P = 0.57).
Radiation dose–response results
| . | Belarus . | . | Russia . | . | Ukraine . | . | Combined . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimated total . | Odds . | . | Odds . | . | Odds . | . | Odds . | . | ||||
| dose (mGy) . | Ratioa . | 95% CI . | Ratioa . | 95% CI . | Ratioa . | 95% CI . | Ratioa . | 95% CI . | ||||
| <1.0 | 1.00 | – | 1.00 | – | 1.00 | – | 1.00 | – | ||||
| 1.0–4.999 | 1.28 | (0.60–2.70) | 1.00 | (0.28–3.50) | 1.49 | (0.92–2.43) | 1.46 | (0.998–2.12) | ||||
| ≥5.0 | 1.58 | (0.74–3.36) | 6.00 | (0.45–79.75) | 3.50 | (1.995–6.15) | 2.60 | (1.70–3.96) | ||||
| Loglinear regression coefficient per mGyb (95% CI) | 0.0024 (−0.0082–0.0131) | −0.0027 (−0.0315–0.0261) | 0.0123 (0.0030–0.0215) | 0.0081 (0.0023–0.0139) | ||||||||
| One-tailed P-value | P = 0.33 | P = 0.57 | P = 0.005 | P = 0.0030 | ||||||||
| Estimated ERR/Gyc (95% CI) | 4.09 (NE–37.7) | −4.94 (NE) | 78.8 (22.1–213) | 32.4 (8.78–84.0) | ||||||||
| . | Belarus . | . | Russia . | . | Ukraine . | . | Combined . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimated total . | Odds . | . | Odds . | . | Odds . | . | Odds . | . | ||||
| dose (mGy) . | Ratioa . | 95% CI . | Ratioa . | 95% CI . | Ratioa . | 95% CI . | Ratioa . | 95% CI . | ||||
| <1.0 | 1.00 | – | 1.00 | – | 1.00 | – | 1.00 | – | ||||
| 1.0–4.999 | 1.28 | (0.60–2.70) | 1.00 | (0.28–3.50) | 1.49 | (0.92–2.43) | 1.46 | (0.998–2.12) | ||||
| ≥5.0 | 1.58 | (0.74–3.36) | 6.00 | (0.45–79.75) | 3.50 | (1.995–6.15) | 2.60 | (1.70–3.96) | ||||
| Loglinear regression coefficient per mGyb (95% CI) | 0.0024 (−0.0082–0.0131) | −0.0027 (−0.0315–0.0261) | 0.0123 (0.0030–0.0215) | 0.0081 (0.0023–0.0139) | ||||||||
| One-tailed P-value | P = 0.33 | P = 0.57 | P = 0.005 | P = 0.0030 | ||||||||
| Estimated ERR/Gyc (95% CI) | 4.09 (NE–37.7) | −4.94 (NE) | 78.8 (22.1–213) | 32.4 (8.78–84.0) | ||||||||
Adjusted for matching.
Regression coefficient (βlogl) in loglinear model for odds ratio of disease as function of dose, estimated with adjustment for matching.
ERR/Gy = excess relative risk per Gy (βlin/1000), estimated for linear model for odds ratio of disease as function of dose, estimated with adjustment for matching. 95% CI is based on profile likelihood. NE = not estimable.
Radiation dose–response results
| . | Belarus . | . | Russia . | . | Ukraine . | . | Combined . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimated total . | Odds . | . | Odds . | . | Odds . | . | Odds . | . | ||||
| dose (mGy) . | Ratioa . | 95% CI . | Ratioa . | 95% CI . | Ratioa . | 95% CI . | Ratioa . | 95% CI . | ||||
| <1.0 | 1.00 | – | 1.00 | – | 1.00 | – | 1.00 | – | ||||
| 1.0–4.999 | 1.28 | (0.60–2.70) | 1.00 | (0.28–3.50) | 1.49 | (0.92–2.43) | 1.46 | (0.998–2.12) | ||||
| ≥5.0 | 1.58 | (0.74–3.36) | 6.00 | (0.45–79.75) | 3.50 | (1.995–6.15) | 2.60 | (1.70–3.96) | ||||
| Loglinear regression coefficient per mGyb (95% CI) | 0.0024 (−0.0082–0.0131) | −0.0027 (−0.0315–0.0261) | 0.0123 (0.0030–0.0215) | 0.0081 (0.0023–0.0139) | ||||||||
| One-tailed P-value | P = 0.33 | P = 0.57 | P = 0.005 | P = 0.0030 | ||||||||
| Estimated ERR/Gyc (95% CI) | 4.09 (NE–37.7) | −4.94 (NE) | 78.8 (22.1–213) | 32.4 (8.78–84.0) | ||||||||
| . | Belarus . | . | Russia . | . | Ukraine . | . | Combined . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimated total . | Odds . | . | Odds . | . | Odds . | . | Odds . | . | ||||
| dose (mGy) . | Ratioa . | 95% CI . | Ratioa . | 95% CI . | Ratioa . | 95% CI . | Ratioa . | 95% CI . | ||||
| <1.0 | 1.00 | – | 1.00 | – | 1.00 | – | 1.00 | – | ||||
| 1.0–4.999 | 1.28 | (0.60–2.70) | 1.00 | (0.28–3.50) | 1.49 | (0.92–2.43) | 1.46 | (0.998–2.12) | ||||
| ≥5.0 | 1.58 | (0.74–3.36) | 6.00 | (0.45–79.75) | 3.50 | (1.995–6.15) | 2.60 | (1.70–3.96) | ||||
| Loglinear regression coefficient per mGyb (95% CI) | 0.0024 (−0.0082–0.0131) | −0.0027 (−0.0315–0.0261) | 0.0123 (0.0030–0.0215) | 0.0081 (0.0023–0.0139) | ||||||||
| One-tailed P-value | P = 0.33 | P = 0.57 | P = 0.005 | P = 0.0030 | ||||||||
| Estimated ERR/Gyc (95% CI) | 4.09 (NE–37.7) | −4.94 (NE) | 78.8 (22.1–213) | 32.4 (8.78–84.0) | ||||||||
Adjusted for matching.
Regression coefficient (βlogl) in loglinear model for odds ratio of disease as function of dose, estimated with adjustment for matching.
ERR/Gy = excess relative risk per Gy (βlin/1000), estimated for linear model for odds ratio of disease as function of dose, estimated with adjustment for matching. 95% CI is based on profile likelihood. NE = not estimable.
In order to express the magnitude of the dose–response in a more familiar format, the excess relative risk at 1 Gy (ERR/Gy) was estimated for each republic and all republics combined. For all republics combined the estimated ERR/Gy was 32.4. The ERR/Gy was much larger in Ukraine (78.8) compared with Belarus (4.1) and Russia (−4.94). CIs were very wide and overlapped.
The results for Russia in the upper and lower parts of Table 4 may appear inconsistent, with an odds ratio of 6.0 for the ≥5 mGy dose category, but a negative slope for the dose–response. Such an apparent contradiction can happen with a small number of individually matched case–control sets. Categorizing doses loses information about the magnitudes of differences between matched cases and controls, and in some matched sets even the direction of the difference. For example, if a case and his/her controls are all within the same dose category, they contribute no information to the estimates in the upper part of Table 4, but still contribute to either a positive or negative estimate of the coefficient in the lower part of the table, depending on their doses.
To investigate further the apparent differences in dose–response between Ukraine and the other two republics, variation of the dose–response across the seven study oblasts was also investigated. For this purpose, each case and control was classified according to raion of residence ATA, or mother's raion of residence ATA if the subject was in utero ATA. As shown in Table 5, only for Rovenskaya Oblast in Ukraine was a significant dose–response observed (P = 0.012). The estimated dose–response coefficients were largest for Cherkasskaya and Chernigovskaya Oblasts: 0.24/mGy and 0.060/mGy, respectively, compared with 0.012/mGy for Rovenskaya Oblast. However owing in large part to the very low mean doses for Cherkasskaya and Chernigovskaya Oblasts, their dose–responses were not statistically significant (P = 0.07 for both oblasts). Also shown in Table 5 is the ERR/Gy, estimated using the linear model. Estimates for the Ukraine oblasts are very large relative to those in Belarus, particularly for Rovenskaya and Cherkasskaya Oblasts, but the CIs for these estimates are very wide.
Radiation dose–response results by Oblasta
| . | . | . | . | Loglinear regression coefficient . | . | . | Mean dose (mGy) . | . | Median dose (mGy) . | . | ERR/Gyb . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Republic . | Oblasta . | Cases . | Controls . | Estimate . | 95% CI . | P-value . | Cases . | Controls . | Cases . | Controls . | Estimate . | 95% CI . | ||||
| Belarus | 114 | 221 | 0.0024 | (−0.0082–0.0131) | 0.33 | 12.81 | 11.74 | 5.61 | 5.02 | 4.09 | (NE–37.7) | |||||
| Gomel'skaya | 65 | 131 | 0.0027 | (−0.0092–0.0146) | 0.33 | 17.54 | 15.99 | 8.92 | 8.94 | 4.33 | (NE–48.4) | |||||
| Mogilevskaya | 49 | 90 | 0.0036 | (−0.0209–0.0282) | 0.39 | 6.54 | 5.55 | 1.07 | 0.70 | 3.06 | (NE–115.6) | |||||
| Russia | Bryanskaya | 39 | 78 | −0.0027 | (−0.0315–0.0260) | 0.57 | 9.97 | 10.49 | 1.38 | 1.09 | −4.94 | NE | ||||
| Ukraine | 268 | 536 | 0.0123 | (0.0030–0.0215) | 0.005 | 10.12 | 3.46 | 0.50 | 0.39 | 78.8 | (22.1–213) | |||||
| Cherkasskaya | 53 | 106 | 0.2371 | (−0.0823–0.5564) | 0.07 | 0.92 | 0.64 | 0.47 | 0.38 | 454 | (−58.3–7527) | |||||
| Chernigovskaya | 81 | 162 | 0.0599 | (−0.0212–0.1409) | 0.07 | 2.21 | 1.15 | 0.45 | 0.51 | 141 | (NE–833) | |||||
| Rovenskaya | 53 | 106 | 0.0116 | (0.0015–0.0218) | 0.012 | 36.51 | 8.46 | 0.70 | 0.26 | 656 | (NE–34 720) | |||||
| Zhitomirskaya | 81 | 162 | 0.0096 | (−0.0084–0.0276) | 0.15 | 6.79 | 4.33 | 0.55 | 0.51 | 11.7 | (NE–81.9) | |||||
| . | . | . | . | Loglinear regression coefficient . | . | . | Mean dose (mGy) . | . | Median dose (mGy) . | . | ERR/Gyb . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Republic . | Oblasta . | Cases . | Controls . | Estimate . | 95% CI . | P-value . | Cases . | Controls . | Cases . | Controls . | Estimate . | 95% CI . | ||||
| Belarus | 114 | 221 | 0.0024 | (−0.0082–0.0131) | 0.33 | 12.81 | 11.74 | 5.61 | 5.02 | 4.09 | (NE–37.7) | |||||
| Gomel'skaya | 65 | 131 | 0.0027 | (−0.0092–0.0146) | 0.33 | 17.54 | 15.99 | 8.92 | 8.94 | 4.33 | (NE–48.4) | |||||
| Mogilevskaya | 49 | 90 | 0.0036 | (−0.0209–0.0282) | 0.39 | 6.54 | 5.55 | 1.07 | 0.70 | 3.06 | (NE–115.6) | |||||
| Russia | Bryanskaya | 39 | 78 | −0.0027 | (−0.0315–0.0260) | 0.57 | 9.97 | 10.49 | 1.38 | 1.09 | −4.94 | NE | ||||
| Ukraine | 268 | 536 | 0.0123 | (0.0030–0.0215) | 0.005 | 10.12 | 3.46 | 0.50 | 0.39 | 78.8 | (22.1–213) | |||||
| Cherkasskaya | 53 | 106 | 0.2371 | (−0.0823–0.5564) | 0.07 | 0.92 | 0.64 | 0.47 | 0.38 | 454 | (−58.3–7527) | |||||
| Chernigovskaya | 81 | 162 | 0.0599 | (−0.0212–0.1409) | 0.07 | 2.21 | 1.15 | 0.45 | 0.51 | 141 | (NE–833) | |||||
| Rovenskaya | 53 | 106 | 0.0116 | (0.0015–0.0218) | 0.012 | 36.51 | 8.46 | 0.70 | 0.26 | 656 | (NE–34 720) | |||||
| Zhitomirskaya | 81 | 162 | 0.0096 | (−0.0084–0.0276) | 0.15 | 6.79 | 4.33 | 0.55 | 0.51 | 11.7 | (NE–81.9) | |||||
Oblast of residence ATA, or mother's oblast of residence ATA if subject was in utero ATA.
ERR/Gy = excess relative risk per Gy (βlin/1000), estimated with adjustment for matching. 95% CI is based on profile likelihood. NE = not estimable (all non-estimable lower 95% confidence limits are <0).
Radiation dose–response results by Oblasta
| . | . | . | . | Loglinear regression coefficient . | . | . | Mean dose (mGy) . | . | Median dose (mGy) . | . | ERR/Gyb . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Republic . | Oblasta . | Cases . | Controls . | Estimate . | 95% CI . | P-value . | Cases . | Controls . | Cases . | Controls . | Estimate . | 95% CI . | ||||
| Belarus | 114 | 221 | 0.0024 | (−0.0082–0.0131) | 0.33 | 12.81 | 11.74 | 5.61 | 5.02 | 4.09 | (NE–37.7) | |||||
| Gomel'skaya | 65 | 131 | 0.0027 | (−0.0092–0.0146) | 0.33 | 17.54 | 15.99 | 8.92 | 8.94 | 4.33 | (NE–48.4) | |||||
| Mogilevskaya | 49 | 90 | 0.0036 | (−0.0209–0.0282) | 0.39 | 6.54 | 5.55 | 1.07 | 0.70 | 3.06 | (NE–115.6) | |||||
| Russia | Bryanskaya | 39 | 78 | −0.0027 | (−0.0315–0.0260) | 0.57 | 9.97 | 10.49 | 1.38 | 1.09 | −4.94 | NE | ||||
| Ukraine | 268 | 536 | 0.0123 | (0.0030–0.0215) | 0.005 | 10.12 | 3.46 | 0.50 | 0.39 | 78.8 | (22.1–213) | |||||
| Cherkasskaya | 53 | 106 | 0.2371 | (−0.0823–0.5564) | 0.07 | 0.92 | 0.64 | 0.47 | 0.38 | 454 | (−58.3–7527) | |||||
| Chernigovskaya | 81 | 162 | 0.0599 | (−0.0212–0.1409) | 0.07 | 2.21 | 1.15 | 0.45 | 0.51 | 141 | (NE–833) | |||||
| Rovenskaya | 53 | 106 | 0.0116 | (0.0015–0.0218) | 0.012 | 36.51 | 8.46 | 0.70 | 0.26 | 656 | (NE–34 720) | |||||
| Zhitomirskaya | 81 | 162 | 0.0096 | (−0.0084–0.0276) | 0.15 | 6.79 | 4.33 | 0.55 | 0.51 | 11.7 | (NE–81.9) | |||||
| . | . | . | . | Loglinear regression coefficient . | . | . | Mean dose (mGy) . | . | Median dose (mGy) . | . | ERR/Gyb . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Republic . | Oblasta . | Cases . | Controls . | Estimate . | 95% CI . | P-value . | Cases . | Controls . | Cases . | Controls . | Estimate . | 95% CI . | ||||
| Belarus | 114 | 221 | 0.0024 | (−0.0082–0.0131) | 0.33 | 12.81 | 11.74 | 5.61 | 5.02 | 4.09 | (NE–37.7) | |||||
| Gomel'skaya | 65 | 131 | 0.0027 | (−0.0092–0.0146) | 0.33 | 17.54 | 15.99 | 8.92 | 8.94 | 4.33 | (NE–48.4) | |||||
| Mogilevskaya | 49 | 90 | 0.0036 | (−0.0209–0.0282) | 0.39 | 6.54 | 5.55 | 1.07 | 0.70 | 3.06 | (NE–115.6) | |||||
| Russia | Bryanskaya | 39 | 78 | −0.0027 | (−0.0315–0.0260) | 0.57 | 9.97 | 10.49 | 1.38 | 1.09 | −4.94 | NE | ||||
| Ukraine | 268 | 536 | 0.0123 | (0.0030–0.0215) | 0.005 | 10.12 | 3.46 | 0.50 | 0.39 | 78.8 | (22.1–213) | |||||
| Cherkasskaya | 53 | 106 | 0.2371 | (−0.0823–0.5564) | 0.07 | 0.92 | 0.64 | 0.47 | 0.38 | 454 | (−58.3–7527) | |||||
| Chernigovskaya | 81 | 162 | 0.0599 | (−0.0212–0.1409) | 0.07 | 2.21 | 1.15 | 0.45 | 0.51 | 141 | (NE–833) | |||||
| Rovenskaya | 53 | 106 | 0.0116 | (0.0015–0.0218) | 0.012 | 36.51 | 8.46 | 0.70 | 0.26 | 656 | (NE–34 720) | |||||
| Zhitomirskaya | 81 | 162 | 0.0096 | (−0.0084–0.0276) | 0.15 | 6.79 | 4.33 | 0.55 | 0.51 | 11.7 | (NE–81.9) | |||||
Oblast of residence ATA, or mother's oblast of residence ATA if subject was in utero ATA.
ERR/Gy = excess relative risk per Gy (βlin/1000), estimated with adjustment for matching. 95% CI is based on profile likelihood. NE = not estimable (all non-estimable lower 95% confidence limits are <0).
In an attempt to better understand the finding of a significant dose–response only in the Rovenskaya Oblast, we examined the distribution of cases and controls by raion. In Rovenskaya Oblast, 24 (45%) of the 53 cases lived ATA in relatively heavily contaminated raions in the northern part of the oblast (which also are characterized by higher transfer coefficients of 137Cs activity from soils to foods), compared with only 13 (12%) of the 106 controls. Much of this difference can be attributed to two raions that were not relatively heavily contaminated: Rovenskii, which includes Rivno City; and Zdolbunovskii, in which 10 (19%) of the 53 cases lived ATA, compared with 56 (53%) of 106 controls. This type of pattern was not observed within the other three oblasts in Ukraine, or the two oblasts in Belarus.
Table 6 shows the dose–response results separately by sex, along with the two-sided P-value for the test of significance of dose effect modification by sex, based on the loglinear dose–response model. There was no significant difference between the dose–response in females and males for any republic or all republics combined. Similarly, the radiation dose–response did not vary significantly in relation to history of prior chest fluoroscopy or history of external diagnostic radiation exposures other than head and neck, dental X-ray, and chest fluoroscopy, and there was also no significant trend of increasing or decreasing dose–response with age ATA (data not shown).
Radiation dose–response results by sex
| . | Belarus . | . | Russia . | . | Ukraine . | . | Combined . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | Estimatea . | 95% CI . | Estimatea . | 95% CI . | Estimatea . | 95% CI . | Estimatea . | 95% CI . | ||||
| Female | 0.0088 (P = 0.21) | (−0.0129–0.0305) | −0.0113 (P = 0.69) | (−0.0554–0.0328) | 0.0228 (P = 0.0146) | (0.0023–0.0434) | 0.0126 (P = 0.016) | (0.0011–0.0241) | ||||
| Male | 0.0004 (P = 0.47) | (−0.0119–0.0127) | 0.0573 (P = 0.17) | (−0.0598–0.1744) | 0.0087 (P = 0.0255) | (−0.00004–0.0174) | 0.0065 (P = 0.021) | (0.0003–0.0127) | ||||
| Interactionb | P = 0.51 | P = 0.21 | P = 0.105 | P = 0.19 | ||||||||
| . | Belarus . | . | Russia . | . | Ukraine . | . | Combined . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | Estimatea . | 95% CI . | Estimatea . | 95% CI . | Estimatea . | 95% CI . | Estimatea . | 95% CI . | ||||
| Female | 0.0088 (P = 0.21) | (−0.0129–0.0305) | −0.0113 (P = 0.69) | (−0.0554–0.0328) | 0.0228 (P = 0.0146) | (0.0023–0.0434) | 0.0126 (P = 0.016) | (0.0011–0.0241) | ||||
| Male | 0.0004 (P = 0.47) | (−0.0119–0.0127) | 0.0573 (P = 0.17) | (−0.0598–0.1744) | 0.0087 (P = 0.0255) | (−0.00004–0.0174) | 0.0065 (P = 0.021) | (0.0003–0.0127) | ||||
| Interactionb | P = 0.51 | P = 0.21 | P = 0.105 | P = 0.19 | ||||||||
Estimated dose–response regression parameter βlogl (per mGy); 95% CI = 95% confidence interval for dose–response regression parameter; P = one-sided P-value for increasing dose–response.
Two-sided P-value for significance of dose–effect modification.
Radiation dose–response results by sex
| . | Belarus . | . | Russia . | . | Ukraine . | . | Combined . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | Estimatea . | 95% CI . | Estimatea . | 95% CI . | Estimatea . | 95% CI . | Estimatea . | 95% CI . | ||||
| Female | 0.0088 (P = 0.21) | (−0.0129–0.0305) | −0.0113 (P = 0.69) | (−0.0554–0.0328) | 0.0228 (P = 0.0146) | (0.0023–0.0434) | 0.0126 (P = 0.016) | (0.0011–0.0241) | ||||
| Male | 0.0004 (P = 0.47) | (−0.0119–0.0127) | 0.0573 (P = 0.17) | (−0.0598–0.1744) | 0.0087 (P = 0.0255) | (−0.00004–0.0174) | 0.0065 (P = 0.021) | (0.0003–0.0127) | ||||
| Interactionb | P = 0.51 | P = 0.21 | P = 0.105 | P = 0.19 | ||||||||
| . | Belarus . | . | Russia . | . | Ukraine . | . | Combined . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | Estimatea . | 95% CI . | Estimatea . | 95% CI . | Estimatea . | 95% CI . | Estimatea . | 95% CI . | ||||
| Female | 0.0088 (P = 0.21) | (−0.0129–0.0305) | −0.0113 (P = 0.69) | (−0.0554–0.0328) | 0.0228 (P = 0.0146) | (0.0023–0.0434) | 0.0126 (P = 0.016) | (0.0011–0.0241) | ||||
| Male | 0.0004 (P = 0.47) | (−0.0119–0.0127) | 0.0573 (P = 0.17) | (−0.0598–0.1744) | 0.0087 (P = 0.0255) | (−0.00004–0.0174) | 0.0065 (P = 0.021) | (0.0003–0.0127) | ||||
| Interactionb | P = 0.51 | P = 0.21 | P = 0.105 | P = 0.19 | ||||||||
Estimated dose–response regression parameter βlogl (per mGy); 95% CI = 95% confidence interval for dose–response regression parameter; P = one-sided P-value for increasing dose–response.
Two-sided P-value for significance of dose–effect modification.
Discussion
This is the first reported population-based study of childhood leukaemia among persons exposed to radiation from the Chernobyl accident conducted in the contaminated regions of Ukraine, Belarus, and Russia using a common methodology and based on individual estimates of radiation dose. We report two principal findings. First, the magnitude of the radiation doses received by study participants was relatively low. Median doses in all three countries were <10 mGy, and mean doses were <15 mGy. These doses were much lower than originally expected, although not inconsistent with earlier estimates of average exposure.17,21,22 Second, there was an overall significant increase in leukaemia risk associated with increasing radiation dose to the bone marrow. This association was most evident in Ukraine, where there appeared to be a disproportionate number of controls from less heavily contaminated raions, but was also apparent (but not statistically significant) in Belarus. There was no evidence of a radiation dose–response relationship in Russia, but the number of leukaemia cases in the study from Russia was quite small compared with the other two countries.
It is well established that exposure to ionizing radiation can increase the risk of leukaemia. Most of the evidence derives from studies of external exposure to low-LET radiation at high dose rates. The most comprehensive assessment of radiation-induced leukaemia is from long-term studies of survivors of the atomic bombings in Hiroshima and Nagasaki. However, it is important to recognize that the exposure circumstances in Japan were very different from Chernobyl, most notably regarding dose rate (nearly instantaneous exposure in Japan) and dose level (average doses to A-bomb survivors were ∼0.25 Sv). Based on recent analyses of incidence data,23 the ERR of all types of leukaemia at 1 Sv is estimated to be 4.37. This estimate is higher for persons exposed under age 20 (6.11), and considerably higher when restricting the analysis to cases that develop within 5–10 years from exposure (18.69). Results are similar based on mortality data,24 with an overall ERR per Sv of 4.62. There is strong evidence of non-linearity in the dose–response for leukaemia in the A-bomb survivor data, with estimates of risks at 0.1 Sv being ∼1/20th of those at 1 Sv.24 Estimates of total dose to survivors in Hiroshima, moreover, include neutron contributions of 10–20% (assuming an RBE of 10 for neutrons).22
The other principal source of information regarding the effects of radiation exposure on leukaemia risk in humans comes from studies of persons exposed for medical reasons. Several large follow-up studies have been conducted of cohorts exposed therapeutically to external low-LET radiation, most notably to treat ankylosing spondilitis,25 cervical,26 uterine,27 and breast28 cancer, tinea capitis,29 tuberculosis,30 and benign gynaecological disease.31 These studies are also characterized by doses considerably higher than those considered in the present study (average doses for the studies referenced above range from ∼0.3 to several Sv), and by relatively high dose rates (dose delivered over a very short time). ERR estimates at 1 Sv from these investigations range from 0.127 to 6.0,25 with considerable variation. Owing to the nature of the radiation exposure in these studies, results at 1 Sv are comparable with those at 1 Gy in the present investigation.
In contrast to these findings, no consistent evidence of an increased risk of leukaemia associated with exposure to radiation from Chernobyl has yet been reported.1,17 The one case–control study that reported an excess of leukaemia among persons 0–20 ATA in Ukraine included a small subset of the present study,8 and radiation doses were estimated for only one-third of the cases and a lesser proportion of controls. It is not clear how the selection of cases and controls for dose estimation was done, and whether it was accomplished in an unbiased manner. There is also no consistent evidence from studies of populations with exposure circumstances that might be somewhat similar to those experienced at Chernobyl. Although there is some evidence22 of a possible increase in leukaemia among persons living near the Techa River in the southern Urals who received both internal and external exposures from the nearby Mayak nuclear facility (average dose of ∼0.5 Sv), a number of questions remain regarding the adequacy of the dosimetry and the methods of case ascertainment. Findings from studies of occupational exposures, which are relatively low and more protracted over time, are inconsistent and inconclusive.17 Similarly, studies of populations living in areas with high background radiation levels have not demonstrated a clear increase in the occurrence of leukaemia.32,33
The point estimates of risk from the present study overall and for Ukraine specifically are much larger in magnitude than the range of estimates reported from studies of persons exposed to much larger doses at higher dose rates, but given the wide CIs are not inconsistent with such estimates. No estimates of similar magnitude have been reported from studies of populations exposed to the lower doses or dose rates that characterize the populations exposed around Chernobyl. Thus, given the inconsistency of the present findings with existing estimates of radiation-associated leukaemia, and the lack of consistency of findings across the three countries, additional analyses were conducted to investigate the potential effects of two aspects of the study methodology that might influence these results. First, we repeated the analysis after excluding cases diagnosed within 5 years after the Chernobyl accident to allow for a period of latency after the start of exposure, as there is some evidence to suggest that the peak occurrence of leukaemia after radiation exposure is ∼5 years.24 The dose–response results were not materially changed by excluding the first 5 years' cases from those in Tables 4 and 5. In fact the estimated radiation dose–response did not vary significantly as the interval of time from the accident to the diagnosis of leukaemia increased (Table 7).
Radiation dose–response results by year of diagnosis
| . | Belarus . | . | Russia . | . | Ukraine . | . | Combined . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year of diagnosis . | Estimatea . | 95% CI . | Estimatea . | 95% CI . | Estimatea . | 95% CI . | Estimatea . | 95% CI . | ||||
| 1986–90 | 0.0072 (P = 0.17) | (−0.0074–0.0217) | −0.1103 (P = 0.73) | (−0.4636–0.2430) | 0.0129 (P = 0.023) | (0.0002–0.0256) | 0.0103 (P = 0.012) | (0.0014–0.0193) | ||||
| 1991–95 | −0.0056 (P = 0.76) | (−0.0215–0.0102) | −0.0005 (P = 0.52) | (−0.0227–0.0217) | 0.0127 (P = 0.057) | (−0.0030–0.0284) | 0.0046 (P = 0.10) | (−0.0025–0.0117) | ||||
| 1996–2000 | 0.0050 (P = 0.33) | (−0.0168–0.0268) | 0.2177 (P = 0.23) | (−0.3645–0.7998) | 0.0340 (P = 0.14) | (−0.0267–0.0947) | 0.0132 (P = 0.07) | (−0.0047–0.0311) | ||||
| Interactionb | 0.46 | 0.17 | 0.70 | 0.49 | ||||||||
| . | Belarus . | . | Russia . | . | Ukraine . | . | Combined . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year of diagnosis . | Estimatea . | 95% CI . | Estimatea . | 95% CI . | Estimatea . | 95% CI . | Estimatea . | 95% CI . | ||||
| 1986–90 | 0.0072 (P = 0.17) | (−0.0074–0.0217) | −0.1103 (P = 0.73) | (−0.4636–0.2430) | 0.0129 (P = 0.023) | (0.0002–0.0256) | 0.0103 (P = 0.012) | (0.0014–0.0193) | ||||
| 1991–95 | −0.0056 (P = 0.76) | (−0.0215–0.0102) | −0.0005 (P = 0.52) | (−0.0227–0.0217) | 0.0127 (P = 0.057) | (−0.0030–0.0284) | 0.0046 (P = 0.10) | (−0.0025–0.0117) | ||||
| 1996–2000 | 0.0050 (P = 0.33) | (−0.0168–0.0268) | 0.2177 (P = 0.23) | (−0.3645–0.7998) | 0.0340 (P = 0.14) | (−0.0267–0.0947) | 0.0132 (P = 0.07) | (−0.0047–0.0311) | ||||
| Interactionb | 0.46 | 0.17 | 0.70 | 0.49 | ||||||||
Estimated dose–response regression parameter βlogl (per mGy); 95% CI = 95% confidence interval for dose–response regression parameter; P = one-sided P-value for increasing dose–response.
Two-sided P-value for significance of dose effect modification.
Radiation dose–response results by year of diagnosis
| . | Belarus . | . | Russia . | . | Ukraine . | . | Combined . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year of diagnosis . | Estimatea . | 95% CI . | Estimatea . | 95% CI . | Estimatea . | 95% CI . | Estimatea . | 95% CI . | ||||
| 1986–90 | 0.0072 (P = 0.17) | (−0.0074–0.0217) | −0.1103 (P = 0.73) | (−0.4636–0.2430) | 0.0129 (P = 0.023) | (0.0002–0.0256) | 0.0103 (P = 0.012) | (0.0014–0.0193) | ||||
| 1991–95 | −0.0056 (P = 0.76) | (−0.0215–0.0102) | −0.0005 (P = 0.52) | (−0.0227–0.0217) | 0.0127 (P = 0.057) | (−0.0030–0.0284) | 0.0046 (P = 0.10) | (−0.0025–0.0117) | ||||
| 1996–2000 | 0.0050 (P = 0.33) | (−0.0168–0.0268) | 0.2177 (P = 0.23) | (−0.3645–0.7998) | 0.0340 (P = 0.14) | (−0.0267–0.0947) | 0.0132 (P = 0.07) | (−0.0047–0.0311) | ||||
| Interactionb | 0.46 | 0.17 | 0.70 | 0.49 | ||||||||
| . | Belarus . | . | Russia . | . | Ukraine . | . | Combined . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year of diagnosis . | Estimatea . | 95% CI . | Estimatea . | 95% CI . | Estimatea . | 95% CI . | Estimatea . | 95% CI . | ||||
| 1986–90 | 0.0072 (P = 0.17) | (−0.0074–0.0217) | −0.1103 (P = 0.73) | (−0.4636–0.2430) | 0.0129 (P = 0.023) | (0.0002–0.0256) | 0.0103 (P = 0.012) | (0.0014–0.0193) | ||||
| 1991–95 | −0.0056 (P = 0.76) | (−0.0215–0.0102) | −0.0005 (P = 0.52) | (−0.0227–0.0217) | 0.0127 (P = 0.057) | (−0.0030–0.0284) | 0.0046 (P = 0.10) | (−0.0025–0.0117) | ||||
| 1996–2000 | 0.0050 (P = 0.33) | (−0.0168–0.0268) | 0.2177 (P = 0.23) | (−0.3645–0.7998) | 0.0340 (P = 0.14) | (−0.0267–0.0947) | 0.0132 (P = 0.07) | (−0.0047–0.0311) | ||||
| Interactionb | 0.46 | 0.17 | 0.70 | 0.49 | ||||||||
Estimated dose–response regression parameter βlogl (per mGy); 95% CI = 95% confidence interval for dose–response regression parameter; P = one-sided P-value for increasing dose–response.
Two-sided P-value for significance of dose effect modification.
Second, we considered the potential impact on the dose–response results of the method of selecting controls, which was somewhat different in Ukraine than in Belarus or Russia. The exclusion of the case raion for selecting the majority of the controls in Ukraine could potentially result in a systematic tendency to select controls from less contaminated areas than cases, particularly when a case is from a more highly contaminated area and given that there are substantially more raions with lower contamination levels than higher contamination levels in the Ukrainian oblasts included in the study, and may have resulted in an overestimate of leukaemia risk. There is some evidence of this occurring in the Rovenskaya Oblast. The method of selecting controls used in Belarus is less likely to result in a similar tendency because a much higher proportion of the raions in the oblasts included from Belarus are relatively heavily contaminated and because controls were selected based on the population distribution by raion.
Other possible factors that might explain, at least in part, the different results in Ukraine could be different underlying susceptibility to the effects of radiation exposure in the study population or systematic differences in the dose estimates. Variation of susceptibility is unlikely to account for this magnitude of risk difference in a population-based study, and there is no reason to believe that Ukrainian children in general are highly susceptible, or that they include a highly susceptible subpopulation large enough to account for these results. Because a common method is used to estimate individual radiation doses in all three republics, it is also unlikely that there is a systematic difference in dosimetry between Ukraine and the other two countries that could account for these results. This would require that there be differences in the underlying dosimetry data that are inputs to the model, or that dosimetry differences exist within the Ukraine, between Ukrainian cases and controls, or perhaps between raions.
It should also be noted that because radiation doses were very low, and much lower than originally anticipated, the statistical power of the study was substantially reduced. The mean and variance of the estimated doses for all 1256 cases and controls in this study were 8 mGy and 688 mGy2, respectively. This implies that testing whether the dose–response regression parameter βlogl in the loglinear model is greater than zero has power of ∼84% under the alternative hypothesis β = 0.006/mGy, which corresponds to an ERR of 6.1 at 1 Gy (one-sided test at 5% critical level). While this level of statistical power might be acceptable in the context of acute external exposures to relatively high doses, it does not ensure with a high degree of confidence that effects of smaller but, nevertheless, appreciable magnitude (an ERR/Gy of <6.1), as might occur following prolonged exposure to smaller doses, will be detected.
This study has a number of strengths. It is population-based, with a high degree of completeness of case ascertainment, and all cases of leukaemia were independently verified by an expert panel of morphologists and haematologists. It is focused on the effects of exposure during childhood, which is considered to be the period of highest risk of radiation-induced leukaemia,17 and is relatively large (421 cases and nearly twice as many controls). Risk estimates are based on individual estimates of radiation dose from Chernobyl for each study participant. Common and standardized methods of data collection and dose estimation were used in all three countries, and extensive data quality control and auditing procedures were employed.
In summary, this study found that: (i) the magnitude of the radiation doses received by study participants was relatively low; and (ii) that there was an overall significant increase in leukaemia risk associated with increasing radiation dose to the bone marrow, which was most evident in Ukraine. These findings, taken at face value, suggest that prolonged exposure to very low radiation doses may increase leukaemia risk as much as or even more than acute exposure, but are inconsistent with published literature regarding the risk of leukaemia in relation to protracted radiation doses of the very low magnitude observed in this study. The large excess risks and significant dose–response observed in this study might be accounted for, at least in part, by an overestimate of risk in Ukraine. Therefore, we conclude that this study provides no convincing evidence of an increased risk of childhood leukaemia as a result of exposure to Chernobyl radiation, since the extent to which the association found is owing to a sampling-derived bias in Ukraine, rather than a radiation-related excess, is unclear. However given the very low estimated radiation doses and resulting low statistical power, the lack of significant dose–responses in Belarus and Russia also cannot convincingly rule out the possibility of an increase in leukaemia risk at low dose levels.
This work was supported by Grant No. 00014-00-1-0989, issued to Georgetown University from the Office of Naval Research in support of the International Consortium for Research on the Health Effects of Radiation. The contents are solely the responsibility of the authors and do not necessarily represent the views of the Office of Naval Research, Georgetown University. US regulations regarding the use of human subjects in research were complied with through approval and oversight of all activities by Institutional Review Boards at participating US institutions and those established in Belarus, Russia, and Ukraine. We thank Colonel Michael Spiro (USMC, Ret) for his administrative leadership of the ICRHER, Colonel Richard McDonald (USMC, Ret) for logistical and administrative support, and Lisa Anderson for technical and administrative support. Oleg Gvozd and Nikita Schlovski-Kordi provided logistical support in Belarus and Russia, respectively. Alexandra Bondar and Andrey Noschenko were responsible for directing the field team that collected the data in Ukraine, and Pavlo Zamostian was responsible for the Ukrainian component of the development and implementation of the common dosimetry system and the estimation of radiation dose for Ukrainian participants. Leif Peterson and Nicholas Dainiak directed initial phases of the study in Ukraine and Belarus, respectively, and Leon Epstein and Gadi Rennert contributed helpful information regarding the possible migration of eligible cases to Israel. We are indebted to members of the Scientific Advisory Committee for their scientific advice and oversight: David Becker, Andre Bouville, Antone Brooks, Stuart Finch (Chair), Shirley Fry, and Arthur Upton. We especially appreciate the contributions of Shirley Fry, not only for her service on the Scientific Advisory Committee throughout, but for serving as Scientific Director for a period mid-way in the study and as interim US Principal Investigator for the Ukraine project. Additionally, we commend Jack Schull, who was the first Scientific Director and was the Chairman of the Board of Directors following the death of Admiral Zumwalt. We gratefully acknowledge the support of the ICRHER Board of Directors: E. William Cole, H. Lawrence Garrett, William Narva, William Norman, Phillip Robinson, Jack Schull (Chair), and Nancy Wentzler. We especially acknowledge the late Admiral Elmo Zumwalt Jr, for his vision and foresight in establishing the ICRHER and for his unending support of this work.
References
Moysich KB, Menezes RJ, Michalek AM. Chernobyl-related ionising radiation exposure and cancer risk: an epidemiological review.
Parkin DM, Cardis E, Masuyer E et al. Childhood leukaemia following the Chernobyl accident: the European Childhood Leukaemia-Lymphoma Incidence Study (ECLIS).
Parkin DM, Clayton D, Black RJ et al. Childhood leukaemia in Europe after Chernobyl: 5 year follow-up.
Raymond L, Schubert H. The ‘post-Chernobyl’ childhood leukemia study (ECLIS Study).
Ivanov EP, Tolochko G, Lazarev VS, Shuvaeva L. Child leukaemia after Chernobyl.
Ivanov EP, Tolochko GV, Shuvaeva LP, Becker S, Nekolla E, Kellerer AM. Childhood leukemia in Belarus before and after the Chernobyl accident.
Ivanov EP, Tolochko GV, Shuvaeva LP, et al. Infant leukemia in Belarus after the Chernobyl accident.
Noshchenko AG, Moysich KB, Bondar A, Zamostyan PV, Drosdova VD, Michalek AM. Patterns of acute leukaemia occurrence among children in the Chernobyl region.
Prisyazhiuk A, Pjatak OA, Buzanov VA, Reeves GK, Beral V. Cancer in the Ukraine, post-Chernobyl.
Auvinen A, Hakama M, Arvela H et al. Fallout from Chernobyl and incidence of childhood leukaemia in Finland, 1976–92.
Hjalmars U, Kulldorff M, Gustafsson G. Risk of acute childhood leukaemia in Sweden after the Chernobyl reactor accident. Swedish Child Leukaemia Group.
Michaelis J, Kaletsch U, Burkart W, Grosche B. Infant leukaemia after the Chernobyl accident.
Petridou E, Proukakis C, Tong D et al. Trends and geographical distribution of childhood leukemia in Greece in relation to the Chernobyl accident.
Petridou E, Trichopoulos D, Dessypris N et al. Infant leukaemia after in utero exposure to radiation from Chernobyl.
Steiner M, Burkart W, Grosche B, Kaletsch U, Michaelis J. Trends in infant leukaemia in West Germany in relation to in utero exposure due to Chernobyl accident.
Tondel M, Carlsson G, Hardell L et al. Incidence of neoplasms in ages 0–19 y in parts of Sweden with high 137Cs fallout after the Chernobyl accident.
United Nations Scientific Committee on the Effects of Atomic Radiation. Sources, Effects and Risks of Ionizing Radiation, 2000 Report to the General Assembly. New York: United Nations,
Noshchenko AG, Zamostyan PV, Bondar OY, Drozdova VD. Radiation-induced leukemia risk among those aged 0–20 at the time of the Chernobyl accident: a case–control study in the Ukraine.
Mahoney MC, Moysich KM, McCarthy PL Jr et al. Conducting multi-national epidemiology research: challenges and lessons from Chernobyl.
McCarthy PL Jr, Paltiel O, Maslova E et al. Histopathologic verification of acute leukemia (AL) in a cohort of 463 post-Chernobyl patients from Belarus, Russia and Ukraine.
United Nations Scientific Committee on the Effects of Atomic Radiation. Sources, Effects and Risks of Ionizing Radiation, 1988 Report to the General Assembly, Annex D, Exposures from the Chernobyl Accident. New York: United Nations,
United Nations Scientific Committee on the Effects of Atomic Radiation. Sources and Effects of Ionizing Radiation, 1994 Report to the General Assembly. New York: United Nations,
Preston DL, Kusumi S, Tomonaga M et al. Cancer incidence in atomic bomb survivors. Part III. Leukemia, lymphoma and multiple myeloma, 1950–1987.
Pierce DA, Shimizu Y, Preston DL, Vaeth M, Mabuchi K. Studies of the mortality of atomic bomb survivors. Report 12, Part I. Cancer: 1950–1990.
Weiss HA, Darby SC, Doll R. Cancer mortality following X-ray treatment for ankylosing spondylitis.
Boice JD Jr, Blettner M, Kleinerman RA et al. Radiation dose and leukemia risk in patients treated for cancer of the cervix.
Curtis RE, Boice JD Jr, Stovall M et al. Relationship of leukemia risk to radiation dose following cancer of the uterine corpus.
Curtis RE, Boice JD Jr, Stovall M et al. Risk of leukemia after chemotherapy and radiation treatment for breast cancer.
Ron E, Modan B, Boice JD Jr. Mortality after radiotherapy for ringworm of the scalp.
Davis FG, Boice JD Jr, Hrubec Z, Monson RR. Cancer mortality in a radiation-exposed cohort of Massachusetts tuberculosis patients.
Inskip PD, Kleinerman RA, Stovall M et al. Leukemia, lymphoma, and multiple myeloma after pelvic radiotherapy for benign disease.
Tao Z, Cha Y, Sun Q. Cancer mortality in high background radiation area of Yangjiang, China, 1979–1995.