-
PDF
- Split View
-
Views
-
Cite
Cite
Francesco Salvo, Giovanni Polimeni, Ugo Moretti, Anita Conforti, Roberto Leone, Olivia Leoni, Domenico Motola, Giulia Dusi, Achille Patrizio Caputi, Adverse drug reactions related to amoxicillin alone and in association with clavulanic acid: data from spontaneous reporting in Italy, Journal of Antimicrobial Chemotherapy, Volume 60, Issue 1, July 2007, Pages 121–126, https://doi.org/10.1093/jac/dkm111
Close - Share Icon Share
Abstract
To analyse an Italian database of spontaneous reporting of suspected adverse drug reactions in order to compare the safety profile of amoxicillin and amoxicillin/clavulanic acid.
Data were retrieved from the spontaneous reports collected by six Italian regions (the GIF database) from January 1988 to June 2005. Drug utilization data were also available for the two drugs. The comparison between amoxicillin and amoxicillin/clavulanic acid was made using the χ2 or Student's t-test, when appropriate. Disproportionality in reporting of adverse events was assessed using reporting odds ratio methodology.
Up to June 2005, the GIF database collected 37 906 reports, of which 1088 were related to amoxicillin/clavulanic acid and 1095 to amoxicillin. The percentage of skin reactions was statistically higher for amoxicillin (82%) than for amoxicillin/clavulanic acid (76%); on the contrary, the percentage of gastrointestinal, hepatic and haematological reactions was significantly higher for amoxicillin/clavulanic acid (13%, 4% and 2%, respectively) than for amoxicillin (7%, 1% and 1%, respectively). Amoxicillin/clavulanic acid seems to be associated with a higher risk of Stevens–Johnson syndrome, purpura and hepatitis than amoxicillin alone. In particular, the reporting rate of hepatitis is on average 9-fold higher for amoxicillin/clavulanic acid than for amoxicillin.
Analysis shows a different safety profile for the two selected drugs. The combination of amoxicillin/clavulanic acid has been increasingly used in Italy and now represents the most frequently antibiotic prescribed by Italian general practitioners. Given the documented level of inappropriate use of β-lactams in Italy, these results should be taken into account by physicians before prescribing amoxicillin/clavulanic acid to patients.
Introduction
In Italy, β-lactams are among the most prominent antibacterial agents in terms of consumption and expenditure. Amoxicillin is a first choice narrow-spectrum antibiotic; its combination with clavulanic acid is suggested for the treatment of patients with suspected or documented Gram-negative infections caused by β-lactamase-producing organisms. In fact clavulanate, although having no significant antibiotic activity, is able to inactivate β-lactamases, thus preventing penicillin degradation and, consequently, antimicrobial resistance.1 This combination was launched on to the Italian pharmaceutical market in 1988 and, since then, it has been increasingly used, now representing the seventh drug in terms of public health expenditure.2 In addition, in 2005, amoxicillin/clavulanic acid ranked first among antibiotics prescribed by Italian general practitioners (GPs), particularly for the treatment of respiratory tract infections, with a growth of 9.6% compared with 2004.2 Looking at other European countries, public health expenditure for amoxicillin/clavulanic acid shows a large variability, ranking from 5 in Portugal to 175 in Germany.2
Amoxicillin and amoxicillin/clavulanic acid are generally well tolerated, although published studies indicate some differences. In particular, amoxicillin/clavulanic acid has been associated with a higher risk of gastrointestinal adverse reactions and hepatotoxicity.3–8
The aim of this study is to compare the safety profile of amoxicillin/clavulanic acid and amoxicillin, using data from the spontaneous reporting system of six Italian regions from January 1988 to June 2005. Drug utilization data are also available for the two drugs.
Methods
The database of the Italian Interregional Group of Pharmacovigilance (GIF) has been analysed. This database collects all spontaneous reports of suspected adverse drug reactions (ADRs) from six Italian regions: the Veneto and the Provincia Autonoma di Trento (since 1988), Lombardy (since 1993), Sicily (since 1996), Emilia Romagna (since 2000) and Friuli Venezia Giulia (since 2003).
Differently from the National Pharmacovigilance Office, the GIF is more focused on research and educational (rather than regulatory) activities in the field of pharmacovigilance. The involved regions have their own Pharmacovigilance Regional Centre and represent a population of about 24 million inhabitants (43% of the total Italian population); however, more than 60% of all Italian reports come from these regions. Analyses of the GIF database are performed every 6 months, with the aim to identify potential alarm signals.
All reports associated with amoxicillin and amoxicillin/clavulanic acid were selected from the database. Each report was classified according to the WHO criteria for causality assessment and only reports with a ‘certain’, ‘probable’ or ‘possible’ causality assessment were included.9
Drugs were grouped following the Italian ‘Sistema Codifa’ linked to the Anatomical Therapeutic Chemical classification. Reactions were classified according to the WHO Adverse Reaction Terminology and defined as ‘serious’ or ‘non-serious’ events on the basis of the WHO Critical Terms List.10
A report was classified as serious if it concerned a reaction that was fatal or life-threatening, involved or prolonged hospitalization or resulted in persistent or significant disability or incapacity or contained an adverse reaction term included in the WHO Critical Terms List. Both national and regional drug sales data (including unbranded drugs) were supplied by the Institute for Medical Statistics Health. This private company gathers data from different sources, including manufacturers, wholesalers and pharmacies.
Drug consumption data were expressed as defined daily doses (DDD) per 1000 inhabitants per day.
National and regional population data have been obtained from the Italian National Statistics Institute (‘ISTAT’) web site (www.istat.it).
The comparison between amoxicillin and amoxicillin/clavulanic acid was made using the χ2 or Student's t-test, when appropriate. Disproportionality of single adverse events in the database was assessed using reporting odds ratio (ROR) methodology, in which the frequency of the selected reaction related to a single drug is compared with that of the same reaction for all other drugs.11 RORs and their 95% CIs were calculated using SPSS statistical software.
Results
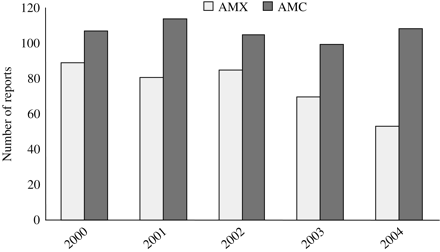
Up to June 2005, the GIF database collected 37 906 reports, 6131 of which were related to vaccines. Reports of amoxicillin/clavulanic acid-related ADRs were 1088, whereas those related to amoxicillin were 1095. Figure 1 shows the number of reports associated with the two drugs during the period 2000–04. The number of reports related to amoxicillin has decreased in the last 2 years.
Number of reports related to amoxicillin/clavulanic acid (AMC) and amoxicillin alone (AMX) in the six Italian regions (Emilia Romagna, Friuli Venezia Giulia, Lombardy, Sicily, the Veneto and the Provincia Autonoma di Trento) over the 5 year period 2000–04.
Table 1 shows the characteristics of the patients with adverse reactions that were related to amoxicillin or amoxicillin/clavulanic acid. No significant difference in the following considered parameters has been found in the two groups: sex distribution, average age, number of patients with concomitant drugs and number of reports in paediatric age. The two groups are also comparable regarding the source of reports, the reporter's category and the administration route. However, amoxicillin/clavulanic acid showed a higher proportion of serious adverse reactions (P < 0.05). Almost all the reports were sent by physicians (60% by hospital physicians, 39% by GPs and 1% by pharmacists), with no significant differences among the drugs considered. Data were homogeneous also regarding the distribution of reporters in the six regions involved.
Characteristics of the two groups of patients affected by ADRs related to amoxicillin/clavulanic acid (AMC) or amoxicillin (AMX) use, during the study period (January 1988–June 2005)
| Characteristics of patients . | AMC . | AMX . | P value . |
|---|---|---|---|
| Number of reports | 1088 | 1095 | |
| Age, years (average ± SD) | 43 ± 23.9 | 41.7 ± 22.9 | 0.195 |
| Women (%) | 60.8 | 63.0 | 0.297 |
| Comedications (%) | 16 | 18 | 0.236 |
| Number of patients < 15 years | 142 | 141 | 0.954 |
| Serious adverse reaction (%) | 39 | 34 | 0.017 |
| Characteristics of patients . | AMC . | AMX . | P value . |
|---|---|---|---|
| Number of reports | 1088 | 1095 | |
| Age, years (average ± SD) | 43 ± 23.9 | 41.7 ± 22.9 | 0.195 |
| Women (%) | 60.8 | 63.0 | 0.297 |
| Comedications (%) | 16 | 18 | 0.236 |
| Number of patients < 15 years | 142 | 141 | 0.954 |
| Serious adverse reaction (%) | 39 | 34 | 0.017 |
Characteristics of the two groups of patients affected by ADRs related to amoxicillin/clavulanic acid (AMC) or amoxicillin (AMX) use, during the study period (January 1988–June 2005)
| Characteristics of patients . | AMC . | AMX . | P value . |
|---|---|---|---|
| Number of reports | 1088 | 1095 | |
| Age, years (average ± SD) | 43 ± 23.9 | 41.7 ± 22.9 | 0.195 |
| Women (%) | 60.8 | 63.0 | 0.297 |
| Comedications (%) | 16 | 18 | 0.236 |
| Number of patients < 15 years | 142 | 141 | 0.954 |
| Serious adverse reaction (%) | 39 | 34 | 0.017 |
| Characteristics of patients . | AMC . | AMX . | P value . |
|---|---|---|---|
| Number of reports | 1088 | 1095 | |
| Age, years (average ± SD) | 43 ± 23.9 | 41.7 ± 22.9 | 0.195 |
| Women (%) | 60.8 | 63.0 | 0.297 |
| Comedications (%) | 16 | 18 | 0.236 |
| Number of patients < 15 years | 142 | 141 | 0.954 |
| Serious adverse reaction (%) | 39 | 34 | 0.017 |
The causality assessment shows that 61% versus 64% of reported adverse reactions were possible and 38% versus 34% were probable for amoxicillin and amoxicillin/clavulanic acid, respectively; 1% were certain for both drugs.
Table 2 shows the consumption of the two drugs in the study area. During the study period, amoxicillin was used more (5.71 DDD/1000 inhabitants/day) than amoxicillin/clavulanic acid (5.04 DDD/1000 inhabitants/day). When single regions were considered, it was found that amoxicillin was used more in Emilia Romagna, Lombardy, Sicily and the Provincia Autonoma di Trento, whereas amoxicillin/clavulanic acid was used more in Friuli Venezia Giulia. However, the differences were small: in the Veneto, drug use was similar for the two drugs. Furthermore, the two drugs were comparable in terms of DDD/inhabitant/day between the GIF regions and the whole of Italy.
Amoxicillin/clavulanic acid (AMC) and amoxicillin (AMX) consumption in the single and grouped GIF regions, during the 5 year period from January 2000 to December 2004
| Area . | AMC (DDD/1000 inhabitants/day) . | AMX (DDD/1000 inhabitants/day) . |
|---|---|---|
| Emilia Romagna | 4.95 | 5.33 |
| Friuli Venezia Giulia | 4.22 | 3.39 |
| Lombardy | 5.34 | 6.77 |
| Sicily | 5.45 | 5.93 |
| The Provincia Autonoma di Trento | 4.28 | 4.91 |
| The Veneto | 4.37 | 4.37 |
| Whole area | 5.04 | 5.71 |
| Whole of Italy | 5.23 | 5.84 |
| Area . | AMC (DDD/1000 inhabitants/day) . | AMX (DDD/1000 inhabitants/day) . |
|---|---|---|
| Emilia Romagna | 4.95 | 5.33 |
| Friuli Venezia Giulia | 4.22 | 3.39 |
| Lombardy | 5.34 | 6.77 |
| Sicily | 5.45 | 5.93 |
| The Provincia Autonoma di Trento | 4.28 | 4.91 |
| The Veneto | 4.37 | 4.37 |
| Whole area | 5.04 | 5.71 |
| Whole of Italy | 5.23 | 5.84 |
Amoxicillin/clavulanic acid (AMC) and amoxicillin (AMX) consumption in the single and grouped GIF regions, during the 5 year period from January 2000 to December 2004
| Area . | AMC (DDD/1000 inhabitants/day) . | AMX (DDD/1000 inhabitants/day) . |
|---|---|---|
| Emilia Romagna | 4.95 | 5.33 |
| Friuli Venezia Giulia | 4.22 | 3.39 |
| Lombardy | 5.34 | 6.77 |
| Sicily | 5.45 | 5.93 |
| The Provincia Autonoma di Trento | 4.28 | 4.91 |
| The Veneto | 4.37 | 4.37 |
| Whole area | 5.04 | 5.71 |
| Whole of Italy | 5.23 | 5.84 |
| Area . | AMC (DDD/1000 inhabitants/day) . | AMX (DDD/1000 inhabitants/day) . |
|---|---|---|
| Emilia Romagna | 4.95 | 5.33 |
| Friuli Venezia Giulia | 4.22 | 3.39 |
| Lombardy | 5.34 | 6.77 |
| Sicily | 5.45 | 5.93 |
| The Provincia Autonoma di Trento | 4.28 | 4.91 |
| The Veneto | 4.37 | 4.37 |
| Whole area | 5.04 | 5.71 |
| Whole of Italy | 5.23 | 5.84 |
Indications for drug use were gathered from the reporting forms (in the section giving the ‘reason for prescribing the drug’), when available. Both drugs were mainly prescribed for respiratory tract infections (43% for amoxicillin versus 53% for amoxicillin/clavulanic acid) and tooth pathologies (21% for amoxicillin versus 11% for amoxicillin/clavulanic acid). In ∼10% of the reports, this information was omitted.
During the period 2000–04, amoxicillin/clavulanic acid had a higher spontaneous reporting rate (number of reports/1 000 000 DDD/year), compared with amoxicillin (2.11 versus 1.52).
Table 3 shows the number of reports according to each system/organ class involved. Skin was the most frequently involved organ for both drugs (it was implicated in ∼80% of total reports), even though the percentage of serious cutaneous reactions was lower when compared with that of all other drugs in the database (P < 0.001; χ2 test). The two drugs showed some differences in their toxicity profile: the percentage of skin reactions was significantly higher for amoxicillin (82%) than for amoxicillin/clavulanic acid (76%). On the contrary, the percentage of gastrointestinal, hepatic and haematological reactions was significantly higher for amoxicillin/clavulanic acid (13%, 4% and 2%, respectively) than for amoxicillin (7%, 1% and 1%, respectively). No statistical difference has been found in adverse reactions involving the other system/organ classes.
Distribution of ADRs related to amoxicillin (AMX) alone and amoxicillin/clavulanic acid (AMC) according to class of organ or system involved; numbers of reportsa, percentages of total reports and percentages of serious reactions are reported for each class (reactions have been considered serious if present in the WHO Critical Terms List)
| Organ or system . | AMC (1088 reports) . | AMX (1095 reports) . | P value . | ||||
|---|---|---|---|---|---|---|---|
| n . | Percentage . | n Serious (%) . | n . | Percentage . | n Serious (%) . | ||
| Skin | 823 | 76 | 66 (8) | 901 | 82 | 81 (9) | <0.0001 |
| Gastrointestinal system | 138 | 13 | 17 (12) | 82 | 7 | 17 (21) | <0.0001 |
| Liver and biliary system | 40 | 4 | 28 (70) | 6 | 1 | 4 (67) | <0.0001 |
| Haematological | 23 | 2 | 22 (96) | 10 | 1 | 10 (100) | 0.021 |
| Body as a whole | 83 | 8 | 39 (47) | 96 | 9 | 41 (43) | 0.332 |
| Urinary and reproductive | 32 | 3 | 26 (81) | 35 | 3 | 30 (86) | 0.729 |
| Respiratory system | 33 | 3 | 19 (58) | 28 | 3 | 15 (54) | 0.499 |
| Cardiovascular system | 19 | 2 | 10 (53) | 19 | 2 | 6 (32) | 0.984 |
| Othersb | 13 | 1 | 1 (8) | 8 | 1 | 1 (13) | 0.266 |
| Organ or system . | AMC (1088 reports) . | AMX (1095 reports) . | P value . | ||||
|---|---|---|---|---|---|---|---|
| n . | Percentage . | n Serious (%) . | n . | Percentage . | n Serious (%) . | ||
| Skin | 823 | 76 | 66 (8) | 901 | 82 | 81 (9) | <0.0001 |
| Gastrointestinal system | 138 | 13 | 17 (12) | 82 | 7 | 17 (21) | <0.0001 |
| Liver and biliary system | 40 | 4 | 28 (70) | 6 | 1 | 4 (67) | <0.0001 |
| Haematological | 23 | 2 | 22 (96) | 10 | 1 | 10 (100) | 0.021 |
| Body as a whole | 83 | 8 | 39 (47) | 96 | 9 | 41 (43) | 0.332 |
| Urinary and reproductive | 32 | 3 | 26 (81) | 35 | 3 | 30 (86) | 0.729 |
| Respiratory system | 33 | 3 | 19 (58) | 28 | 3 | 15 (54) | 0.499 |
| Cardiovascular system | 19 | 2 | 10 (53) | 19 | 2 | 6 (32) | 0.984 |
| Othersb | 13 | 1 | 1 (8) | 8 | 1 | 1 (13) | 0.266 |
aA report may contain multiple system reactions; more events related to the same system/organ class have been counted as one event.
bNone of the other organ systems involved accounted for more than 1% of reports.
Distribution of ADRs related to amoxicillin (AMX) alone and amoxicillin/clavulanic acid (AMC) according to class of organ or system involved; numbers of reportsa, percentages of total reports and percentages of serious reactions are reported for each class (reactions have been considered serious if present in the WHO Critical Terms List)
| Organ or system . | AMC (1088 reports) . | AMX (1095 reports) . | P value . | ||||
|---|---|---|---|---|---|---|---|
| n . | Percentage . | n Serious (%) . | n . | Percentage . | n Serious (%) . | ||
| Skin | 823 | 76 | 66 (8) | 901 | 82 | 81 (9) | <0.0001 |
| Gastrointestinal system | 138 | 13 | 17 (12) | 82 | 7 | 17 (21) | <0.0001 |
| Liver and biliary system | 40 | 4 | 28 (70) | 6 | 1 | 4 (67) | <0.0001 |
| Haematological | 23 | 2 | 22 (96) | 10 | 1 | 10 (100) | 0.021 |
| Body as a whole | 83 | 8 | 39 (47) | 96 | 9 | 41 (43) | 0.332 |
| Urinary and reproductive | 32 | 3 | 26 (81) | 35 | 3 | 30 (86) | 0.729 |
| Respiratory system | 33 | 3 | 19 (58) | 28 | 3 | 15 (54) | 0.499 |
| Cardiovascular system | 19 | 2 | 10 (53) | 19 | 2 | 6 (32) | 0.984 |
| Othersb | 13 | 1 | 1 (8) | 8 | 1 | 1 (13) | 0.266 |
| Organ or system . | AMC (1088 reports) . | AMX (1095 reports) . | P value . | ||||
|---|---|---|---|---|---|---|---|
| n . | Percentage . | n Serious (%) . | n . | Percentage . | n Serious (%) . | ||
| Skin | 823 | 76 | 66 (8) | 901 | 82 | 81 (9) | <0.0001 |
| Gastrointestinal system | 138 | 13 | 17 (12) | 82 | 7 | 17 (21) | <0.0001 |
| Liver and biliary system | 40 | 4 | 28 (70) | 6 | 1 | 4 (67) | <0.0001 |
| Haematological | 23 | 2 | 22 (96) | 10 | 1 | 10 (100) | 0.021 |
| Body as a whole | 83 | 8 | 39 (47) | 96 | 9 | 41 (43) | 0.332 |
| Urinary and reproductive | 32 | 3 | 26 (81) | 35 | 3 | 30 (86) | 0.729 |
| Respiratory system | 33 | 3 | 19 (58) | 28 | 3 | 15 (54) | 0.499 |
| Cardiovascular system | 19 | 2 | 10 (53) | 19 | 2 | 6 (32) | 0.984 |
| Othersb | 13 | 1 | 1 (8) | 8 | 1 | 1 (13) | 0.266 |
aA report may contain multiple system reactions; more events related to the same system/organ class have been counted as one event.
bNone of the other organ systems involved accounted for more than 1% of reports.
As regards serious skin reactions, a higher proportion of Stevens–Johnson syndrome (SJS) related to amoxicillin/clavulanic acid (12 versus 3 for amoxicillin) was found, and a higher number of angioedema related to amoxicillin (46 versus 24 for amoxicillin/clavulanic acid). A case of toxic epidermal necrolysis (TEN) associated with amoxicillin/clavulanic acid was also reported.
Significant differences related to the seriousness of reactions to amoxicillin/clavulanic acid versus amoxicillin were found only for hepatobiliary (28 versus 4) and haematological systems (22 versus 10). Serious hepatic reactions related to amoxicillin/clavulanic acid included 16 cholestatic hepatitis, 11 hepatitis, 1 hepatic necrosis and 1 hepatocellular damage, whereas those related to amoxicillin included 2 cholestatic hepatitis, 1 hepatitis and 1 hepatic necrosis. None of these reactions had a fatal outcome. Serious haematological reactions related to amoxicillin/clavulanic acid included purpura (14), pancytopenia (3), thrombocytopenia (3), agranulocytosis (2), granulocytopenia (1), leucopenia (3) and medullary aplasia (1). Serious haematological reactions related to amoxicillin included purpura (6), thrombocytopenia (3), granulocytopenia (2) and leucopenia (1).
The higher proportion of serious hepatic reactions and SJS associated with amoxicillin/clavulanic acid emerged also from the ROR analysis. The ROR for serious hepatic reactions related to amoxicillin/clavulanic acid was 1.17 (95% CI 0.78–1.75), whereas the ROR for amoxicillin was 0.13 (95% CI 0.03–0.24). The ROR for amoxicillin/clavulanic acid-related SJS was 2.6 (95% CI 1.37–4.64), whereas the ROR for amoxicillin was 0.64 (95% CI 0.16–2.08).
Discussion
The analysis of the GIF spontaneous reporting database suggests that in the real-life situation, the safety profile of amoxicillin is better than that of amoxicillin/clavulanic acid. Weaknesses of this methodology are well documented.12 First, to report an ADR, a physician must suspect a drug–event association, which can be hard to assess, especially for previously unknown events. Secondly, a definite association cannot be provided on the basis of simple suspicion, but requires complementary post-marketing safety assessment methods, such as cohort and case–control studies. Finally, this methodology does not allow the quantification of risks, because adverse events are always under-reported. However, when drug consumption data are available and the reporting rate can be assumed to be more or less of the same magnitude for the reference drugs, it is possible to compare the safety profile of a group of drugs in the same therapeutic class.13
Differently from other European countries, in Italy amoxicillin/clavulanic acid prescription showed an increasing trend from 2002 to 2005 (from 4.5 to 6.2 DDD/1000 inhabitants/day), whereas amoxicillin prescription remained relatively constant over the same time (from 4.2 to 4.1 DDD/1000 inhabitants/day).2,14–16 Several studies have already shown that broad-spectrum antibiotics are often indiscriminately prescribed;17,18 in particular, an observational study showed that only 40% of first-choice antibiotics are appropriately prescribed by Italian GPs.19 Other studies highlighted that, although microbial resistance is not a major problem,20–22 Italian GPs often prescribe a β-lactamase-resistant antibiotic.19 Confirmation of this attitude was not possible, as reasons for prescribing the two antibiotics were often absent or incomplete in our reports.
Reports of adverse reactions collected in the GIF database would seem to show that the higher reporting rate associated with amoxicillin/clavulanic acid when compared with amoxicillin during the period 2000–04 (2.11 versus 1.52) is attributable to a higher risk for the former. This finding supports several previous studies, showing a greater toxicity with amoxicillin/clavulanic acid when compared with amoxicillin.3–8,23,24
The age and sex of patients involved in ADRs did not significantly differ between the two drugs. In accordance with the findings of other drug surveillance systems, women seem to be more likely to develop ADRs in both groups.25–27
Analysis shows that differences between the two drugs were found for cutaneous, gastrointestinal, liver and haematological reactions. Skin was the most affected system for both drugs, with a higher frequency for amoxicillin (P < 0.05). This may reflect a relative over-reporting of skin reactions, which are often easier to recognize than those involving other organs.25 No statistical difference was found for serious skin reactions; these results are in line with those reported by van der Linden et al.24 However, when single case reports were considered, the clinical manifestations of severe skin reactions reported in our database showed some differences. In particular, amoxicillin/clavulanic acid showed a higher proportion of SJS–TEN (13 versus 3), whereas angio-oedema was more frequently attributed to amoxicillin (46 versus 24). Results of ROR analysis confirmed this disproportionality for amoxicillin/clavulanic acid-related SJS, although a search through the literature did not reveal other comparative studies on this specific issue, but only some published case reports.28,29
In line with the literature,3,4 it was found that amoxicillin/clavulanic acid is associated with a higher number of reports of gastrointestinal reactions, although these are less serious (12% versus 21%). Two clinical trials found that the frequency of gastrointestinal events is related to the dosage of clavulanic acid.30,31 Moreover, the higher incidence of amoxicillin/clavulanic acid-induced diarrhoea could be explained by its greater resistance to β-lactamases, which may result in a higher concentration of amoxicillin in the intestine and a consequent stronger modification of the bacterial flora. In addition, a double-blind cross-over study demonstrated that amoxicillin/clavulanic acid is often associated with small intestinal motor disturbances.4 Although these effects are usually minor and transient, they may lead to the interruption of treatment; therefore, further studies are necessary to assess the role of clavulanic acid in gastrointestinal disturbances.
Spontaneous reports from the given database showed that amoxicillin/clavulanic acid use is associated with a higher frequency of liver damage when compared with amoxicillin. Although ROR analysis for serious hepatic reactions did not provide significant differences for amoxicillin/clavulanic acid when compared with the entire database of ADRs (1.17; 95% CI 0.78–1.75), it should be noticed that the significant lower value of amoxicillin-induced hepatotoxicity (0.13; 95% CI 0.03–0.24) supports the hypothesis that amoxicillin is essentially a non-hepatotoxic drug.5,32–34 The role of the clavulanate component in the development of liver injury is supported by two published papers in which rechallenge with amoxicillin alone was well tolerated, whereas rechallenge with amoxicillin/clavulanic acid caused a second episode of hepatitis.6,35 In addition, clavulanate combined with other β-lactams can also lead to liver injury.32 Several studies have investigated the nature of hepatitis related to amoxicillin/clavulanic acid.6,7,35–38 Hepatic reactions are described as uncommon and self-limiting;37 their onset is generally delayed from several days up to weeks after the start of the therapy and might also occur several days after its cessation. This is in line with our data, in which amoxicillin/clavulanic acid hepatic reactions occurred on average 8.9 days after the first administration (range 1–48 days). Moreover, in 13 cases, liver injury was diagnosed 1–38 days after drug cessation. Hepatitis caused by amoxicillin/clavulanic acid is mostly reversible, with a wide variability in the duration of symptoms. However, a recent paper by Andrade et al.,39 analysing records from a Spanish hepatotoxicity registry, showed that amoxicillin/clavulanic acid was the most frequent causative drug of chronic liver injury.
The most common clinical manifestation of amoxicillin/clavulanic acid liver injury is cholestatic hepatitis, probably due to immuno-allergy.38,40,41 Men are affected more frequently than women and also age is a generally recognized risk factor.6,35,40 Larrey et al.33 found that hepatotoxicity seems more frequent in older men who take a prolonged course of the drug. On the contrary, Thomson et al.34 did not find a significant correlation between the duration of therapy and hepatitis. An estimate of the incidence of hepatitis related to amoxicillin/clavulanic acid was provided by De Abajo et al.41; this case–control study confirmed that among antibiotics, amoxicillin/clavulanic acid showed the strongest association with liver injury, with no difference for age, sex and duration of treatment. The estimated incidence was close to 1 per 10 000 users, slightly lower than the one previously estimated by Garcia Rodriguez et al. (1.7 per 10 000 users).5
Hautekeete et al.42 found a significant association between a human leucocyte antigen (HLA) haplotype and drug-induced immuno-allergic hepatitis. Their results suggested an ‘immunologic idiosyncrasy’ in the occurrence of amoxicillin/clavulanic acid-induced hepatitis, at least partially mediated through HLA class II antigens; this explanation might be applied also to amoxicillin/clavulanic acid-induced SJS and TEN. Immunogenetic studies on these patients should be carried out to confirm or refute this hypothesis.
Finally, amoxicillin/clavulanic acid was associated with a higher percentage of haematological reactions than amoxicillin. In particular, purpura was the most common blood disorder reported. However, we found little information about haematological reactions related to amoxicillin/clavulanic acid.
Prolongation of prothrombin time, purpura, thrombocytopenia, agranulocytosis, granulocytopenia and leucopenia are described as uncommon side effects in the Summary of Product Characteristics of amoxicillin/clavulanic acid.1 Several case reports of transient neutropenia and purpura43–47 and one case of severe neutropenia related to a prolonged treatment with orally administered amoxicillin/clavulanic acid have been published.45 Antibiotics are known to cause hypoprothrombinaemia as a consequence of killing the intestinal bacteria, which are a source of activated vitamin K, a necessary co-factor in the synthesis of four of the clotting factors.48 As for diarrhoea, this mechanism might explain the higher proportion of amoxicillin/clavulanic acid-induced purpura than amoxicillin. It should be considered that purpura may also be caused by vasculitis49 and therefore may not have a haematological origin; however, clinical data available from the spontaneous reporting forms failed to provide any element to clarify the pathogenesis of this adverse reaction.
Conclusions
Analysis supports the evidence of a different safety profile for the two selected drugs. Amoxicillin/clavulanic acid was associated with a higher spontaneous reporting rate (2.11 versus 1.52 reports/1 000 000 DDD/year) and a higher number of serious adverse reaction (e.g. SJS, purpura and hepatotoxicity). Liver injuries, in particular, were 9-fold more frequent for amoxicillin/clavulanic acid when compared with amoxicillin.
These findings should encourage physicians to perform an accurate risk/benefit evaluation before prescribing amoxicillin/clavulanic acid to individual patients, favouring amoxicillin for first-choice treatment of non-complicated infectious diseases, as suggested by several guidelines.50–56
By prescribing more appropriately, further benefits might be obtained by the National Health System, not only in terms of minor risks of adverse reactions for patients but also in terms of costs, given the heavier economic burden of amoxicillin/clavulanic acid when compared with amoxicillin.
Acknowledgements
We are very grateful to the Pharmaceutical Departments of Emilia Romagna, Friuli Venezia Giulia, Lombardy, Sicily and the Veneto and their local Health Districts, for collecting the adverse reaction forms. No financial support has been received for this study.
Transparency declarations
None to declare.