-
PDF
- Split View
-
Views
-
Cite
Cite
Petra Warschburger, Johanna Hänig, Michael Friedt, Carsten Posovszky, Maike Schier, Claudia Calvano, Health-Related Quality of Life in Children With Abdominal Pain Due to Functional or Organic Gastrointestinal Disorders, Journal of Pediatric Psychology, Volume 39, Issue 1, January/February 2014, Pages 45–54, https://doi.org/10.1093/jpepsy/jst070
Close - Share Icon Share
Abstract
Objective Comparing health-related quality of life (HRQOL) in children suffering from functional and organic gastrointestinal disorders and to identify predictors for HRQOL. Methods Children with functional (n = 70) and organic (n = 100) gastrointestinal disorders, aged 8–18 years and referred to a pediatric gastroenterologist, completed questionnaires assessing pain severity, coping, and HRQOL. Results The sample reported low HRQOL scores, even significantly lower compared with reference values of chronically ill children, derived from normative data of KINDL-R, a generic QOL questionnaire. HRQOL was not significantly associated with age, gender, duration of pain, and diagnosis (functional gastrointestinal disorder vs. organic gastrointestinal disorder). Pain severity and catastrophizing were significantly associated with HRQOL, with catastrophizing fully mediating the relationship between pain and HRQOL. Conclusion The emotional burden associated with chronic abdominal pain—regardless of its cause—is enormous. Interventions should target the children’s coping strategies, as catastrophizing seems to be the causal link between pain and HRQOL.
Introduction
Chronic abdominal pain (CAP) is one of the most prevalent conditions in childhood and adolescence (Du, Knopf, Zhuang, & Ellert, 2011). Chronic or recurrent episodes of abdominal pain can be explained as symptom of an underlying organic disease [e.g., inflammatory bowel disease (IBD)] or can be defined as functional abdominal pain (FAP; Di Lorenzo et al., 2005) when infectious, inflammatory, structural, or biochemical processes are absent. This study provides a systematic analysis of health-related quality of life (HRQOL) in children suffering from functional gastrointestinal disorders (FGID) and children suffering from organic gastrointestinal disorders (OGID). Considering the large variability in phenomenology, the use of one consistent terminology for FAP is necessary. Standardized diagnostic criteria by the Rome III criteria now state FAP as FGID with a minimum duration of pain for 2 months with at least one pain episode per week (Rasquin et al., 2006). However, in previous and current research, the term “FAP” is often used with various and diffuse definitions. Prevalence rates vary on account of these different definitions: In a systematic review, King et al. (2011) reported that 5–22.2% of children and adolescents report weekly episodes of abdominal pain. FAP poses a considerable burden on the children and their families. Untreated, FAP is highly persistent into adulthood (Jarrett, Heitkemper, Czyzewski, & Shulman, 2003). FAP can be associated with increased psychological symptoms, poor school attendance, and a high rate of health care utilization (Saps et al., 2009). In accordance with current guidelines and the Rome III process (Rasquin et al., 2006), the term FGID will be used instead of FAP.
The assessment of HRQOL is well-established in research as a measure for the overall psychosocial functioning of chronically ill children and adolescents and as a major outcome variable for interventions (Palermo et al., 2008). The influence of pain on quality of life (QOL) has been reported many times: Previous research consistently shows that HRQOL of children suffering from chronic pain is reduced compared with healthy children (Gold et al., 2009; Petersen, Hägglöf, & Bergström, 2009). Until now, little is known about the differences in HRQOL of FAP compared with other diagnoses. Whereas Gold et al. (2009) reported lower HRQOL scores compared with published data on children with cancer and rheumatoid conditions, Varni, Limbers, and Burwinkle (2007) found higher HRQOL scores. Despite the high prevalence of FGID, the majority of studies analyzing HRQOL concentrate on children and adolescents either with OGIDs like IBD (e.g., Crohn’s disease; Greenley, Kunz, Schurman, & Swanson, 2013; Hill et al., 2010; Otley et al., 2006) or chronic pain in general (Huguet, Eccleston, Miró, & Gauntlett-Gilbert, 2009; Merlijn et al., 2006; Petersen et al., 2009; Saps et al., 2009), whereas studies focusing explicitly on FGID as defined by Rome criteria are rather scarce (Varni et al., 2006; Youssef, Murphy, Langseder, & Rosh, 2006).
To date, only two studies compared the QOL of children with FGID with those with OGID. Youssef et al. (2006) contrasted children with FAP with children suffering from IBD or gastroesophageal reflux disease. Varni et al. (2006) compared pediatric patients with irritable bowel syndrome (IBS), FAP (Rome II criteria), and OGID. In both studies, the HRQOL in children with FAP did not differ from the other groups. To our knowledge, no studies have examined the implications for HRQOL of children suffering from FGID defined by Rome III criteria so far. Therefore, it remains unclear whether the existing results can be transferred to the newly defined diagnostic categories as well.
The findings on demographic influences on children’s QOL are rather controversial. In relation to age, most studies reported no influence (Merlijn et al., 2006), whereas others found a higher QOL in younger children as well as gender-specific differences (Petersen et al., 2009). The severity of disease (often defined as intensity and frequency of pain symptoms) was associated with low HRQOL in many studies (Greenley et al., 2013; Otley et al., 2006; Varni et al., 2006). According to Petersen et al. (2009), group differences reached moderate to high effect sizes (Cohen’s d = .63–.73).
It has consistently been found that coping strategies are more important than demographic or disease-related parameters for adaption to chronic diseases. Strategies are often divided into adaptive (e.g., positive self-instruction, acceptance, and distraction) and maladaptive (e.g., rumination, passivity, avoidance, and especially catastrophizing) ones (Walker, Baber, Garber, & Smith, 2008). The tendency to catastrophize has been investigated in recent studies, proving to be maladaptive in many ways: Associations with pain intensity, disability, as well as HRQOL (Huguet et al., 2009; van der Zaag-Loonen, Grootenhuis, Last, & Derkx, 2004) were found. Huguet et al. (2009) showed that a catastrophizing coping style partially mediates between experience of pain and HRQOL in children with chronic pain, whereas Merlijn et al. (2006) pointed out that catastrophizing enforces the negative association between pain intensity and HRQOL. There is consistent evidence underscoring the relevance of catastrophizing, but the exact mode of action is still unclear. To our knowledge, there are no further studies on the influence of coping on HRQOL in children with CAP.
Therefore, with a sample of children and adolescents reporting CAP, the aims of this study were to (1) systematically compare FGID with OGID with respect to HRQOL and with respect to normative data of healthy and chronically ill children derived from KINDL-R (Ravens-Sieverer & Bullinger, 2000), and (2) to find predictors of HRQOL by analyzing the mediating influences of coping variables on HRQOL. It was hypothesized that: (1) HRQOL is comparable in children with FGID and OGID; (2) HRQOL is lower compared with healthy children but does not differ from other chronic diseases; (3) that beyond demographic influences, maladaptive coping strategies would be associated with a poorer HRQOL; and (4) catastrophizing will mediate the relationship between pain and HRQOL.
Method
Participants
One hundred seventy children and adolescents (aged 8–18 years) consulted a pediatric gastroenterologist due to CAP in the participating study centers. Participants included n = 70 children with FGID (58.6% female) and n = 100 children with OGID (68.0% female). Application of the Rome III classification for functional pain-related GIDs to the FGID group resulted in the highest proportion of FAP (45.7%), of which 71.88% fulfilled the criteria for functional abdominal pain syndrome. IBS and functional dyspepsia were reported in 31.4 and 20.0% of cases, respectively. In the OGID group, 28% of children suffered from inflammatory diseases (M. Crohn 10.6%, ulcerative colitis 2.1%, duodenitis 2.1%, esophagitis/gastritis, pancreatitis, esophageal reflux, ulcer, celiac disease, 1.1% each; more than one inflammatory disease 3.2%), whereas 72% suffered from food intolerances and motility disorders (lactose intolerance 13.0%, fructose malabsorption 41.3%, other food intolerances 3.3%, lactose and fructose intolerance 12.0%, constipation 11.0%). Table I summarizes demographic characteristics of the sample. FGID and OGID did not significantly differ with respect to age [t (168) = 1.93, p = .055], gender [χ2 (1) = 1.59, p = .207], familial wealth [t (162) = −.41, p = .684], and number of medical visits in the past 6 months [t (156) = −.257, p = .798]. The proportion of single-parent families [χ2 (1) = .18, p = .673] and full-time working parent [χ2 (1) = 1.25, p = .264] and the level of educational background [χ2 (2) = 2.45, p = .294] were comparable across groups. In addition, pain characteristics [pain severity (PS), duration since onset] in the sample were compared between children suffering from FGID or OGID. With respect to PS, that is, the sum of pain intensity and frequency, children in the FGID group reported higher scores [t (164.63) = 2.83, p = .005]. PS will be included as a covariate in analyses of variance.
Sample Characteristics
| . | FGID (n = 70) . | OGID (n = 100) . | . | . | ||
|---|---|---|---|---|---|---|
| Variables . | n . | M (SD) . | n . | M (SD) . | t (df)/χ2 (df) . | p . |
| Age | 70 | 12.13 (2.27) | 100 | 11.40 (2.52) | t (168) = 1.93 | .055 |
| Pain severity | 70 | 7.11 (2.14) | 99 | 6.06 (2.69) | t (164.63) = 2.83 | .005* |
| Family Affluence Scale | 66 | 5.76 (1.67) | 98 | 5.87 (1.70) | t (162) = −.41 | .684 |
| Medical visits in past 6 months | 65 | 4.38 (3.42) | 92 | 4.22 (4.21) | t (156) = −.257 | .798 |
| Gender n (female/male) | 41/29 | 68/32 | χ2 (1) = 1.59 | .207 | ||
| Single parent n (yes/no) | 13/57 | 21/78 | χ2 (1) = .18 | .673 | ||
| Education n (low/medium/high) | 15/27/28 | 13/47/40 | χ2 (2) = 2.45 | .294 | ||
| Full-time working n (full/part) | 25/22 | 50/29 | χ2 (1) = 1.25 | .264 | ||
| . | FGID (n = 70) . | OGID (n = 100) . | . | . | ||
|---|---|---|---|---|---|---|
| Variables . | n . | M (SD) . | n . | M (SD) . | t (df)/χ2 (df) . | p . |
| Age | 70 | 12.13 (2.27) | 100 | 11.40 (2.52) | t (168) = 1.93 | .055 |
| Pain severity | 70 | 7.11 (2.14) | 99 | 6.06 (2.69) | t (164.63) = 2.83 | .005* |
| Family Affluence Scale | 66 | 5.76 (1.67) | 98 | 5.87 (1.70) | t (162) = −.41 | .684 |
| Medical visits in past 6 months | 65 | 4.38 (3.42) | 92 | 4.22 (4.21) | t (156) = −.257 | .798 |
| Gender n (female/male) | 41/29 | 68/32 | χ2 (1) = 1.59 | .207 | ||
| Single parent n (yes/no) | 13/57 | 21/78 | χ2 (1) = .18 | .673 | ||
| Education n (low/medium/high) | 15/27/28 | 13/47/40 | χ2 (2) = 2.45 | .294 | ||
| Full-time working n (full/part) | 25/22 | 50/29 | χ2 (1) = 1.25 | .264 | ||
Note. Education was coded as low (number of degree or special school), medium (secondary school), and high (diploma or university degree). Missing values account for deviations in sums.
*p < .05.
Sample Characteristics
| . | FGID (n = 70) . | OGID (n = 100) . | . | . | ||
|---|---|---|---|---|---|---|
| Variables . | n . | M (SD) . | n . | M (SD) . | t (df)/χ2 (df) . | p . |
| Age | 70 | 12.13 (2.27) | 100 | 11.40 (2.52) | t (168) = 1.93 | .055 |
| Pain severity | 70 | 7.11 (2.14) | 99 | 6.06 (2.69) | t (164.63) = 2.83 | .005* |
| Family Affluence Scale | 66 | 5.76 (1.67) | 98 | 5.87 (1.70) | t (162) = −.41 | .684 |
| Medical visits in past 6 months | 65 | 4.38 (3.42) | 92 | 4.22 (4.21) | t (156) = −.257 | .798 |
| Gender n (female/male) | 41/29 | 68/32 | χ2 (1) = 1.59 | .207 | ||
| Single parent n (yes/no) | 13/57 | 21/78 | χ2 (1) = .18 | .673 | ||
| Education n (low/medium/high) | 15/27/28 | 13/47/40 | χ2 (2) = 2.45 | .294 | ||
| Full-time working n (full/part) | 25/22 | 50/29 | χ2 (1) = 1.25 | .264 | ||
| . | FGID (n = 70) . | OGID (n = 100) . | . | . | ||
|---|---|---|---|---|---|---|
| Variables . | n . | M (SD) . | n . | M (SD) . | t (df)/χ2 (df) . | p . |
| Age | 70 | 12.13 (2.27) | 100 | 11.40 (2.52) | t (168) = 1.93 | .055 |
| Pain severity | 70 | 7.11 (2.14) | 99 | 6.06 (2.69) | t (164.63) = 2.83 | .005* |
| Family Affluence Scale | 66 | 5.76 (1.67) | 98 | 5.87 (1.70) | t (162) = −.41 | .684 |
| Medical visits in past 6 months | 65 | 4.38 (3.42) | 92 | 4.22 (4.21) | t (156) = −.257 | .798 |
| Gender n (female/male) | 41/29 | 68/32 | χ2 (1) = 1.59 | .207 | ||
| Single parent n (yes/no) | 13/57 | 21/78 | χ2 (1) = .18 | .673 | ||
| Education n (low/medium/high) | 15/27/28 | 13/47/40 | χ2 (2) = 2.45 | .294 | ||
| Full-time working n (full/part) | 25/22 | 50/29 | χ2 (1) = 1.25 | .264 | ||
Note. Education was coded as low (number of degree or special school), medium (secondary school), and high (diploma or university degree). Missing values account for deviations in sums.
*p < .05.
Procedure
Recruitment took place in cooperation with pediatric gastroenterologists at 16 study centers throughout Germany. Children and adolescents consulting a pediatric gastroenterologist were eligible for study participation if they met the following inclusion criteria: (1) aged 8–18 years, (2) suffering from chronic or recurrent abdominal pain, (3) adequate German language skills, and (4) participation of one parent with informed consent. After informed consent, both the child and parent filled out a set of validated questionnaires. The attending physician stated the diagnosis for each patient (FGID vs. OGID). In total, 635 pairs of questionnaires were handed out, of which 223 (35.12%) complete pairs were returned. The application of inclusion criteria resulted in a sample size of n = 192. In n = 7, medical diagnosis was missing. By applying the Rome III criteria to the FGID group, 22 children were excluded. The final data set consisted of n = 170 complete dyadic measurements (FGID n = 70, OGID n = 100), which represents a final response rate of 27%. This study was approved by the Ethical Review Board of the University of Potsdam.
Measures
Socioeconomic variables (educational background, marital status, and familial wealth) were assessed by parent report. Familial wealth was measured using the Family Affluence Scale (Boyce, Torsheim, Currie, & Zambon, 2006). The Rome III criteria for pain-related FGIDs (Rasquin et al., 2006) were also collected by parent report (items according to the Questionnaire on Pediatric Gastrointestinal Symptoms, Caplan, Walker, & Rasquin, 2005).
Health-Related Quality of Life
Children’s HRQOL was assessed by self-report using the KINDL-R (Ravens-Sieberer & Bullinger, 2000), which has well-established reliability and validity (Erhart, Ellert, Kurth, & Ravens-Sieberer, 2009). The KINDL-R covers six dimensions of HRQOL: Physical, mental, self-esteem, family, friends, and school. Each item refers to the well-being over the past week, for example, “Last week, I felt ill.” (physical well-being). Likert scaling comprises values 1–5 (never–seldom–sometimes–often–always). In our sample, internal consistency for the total score was acceptable (α = .76). Scale scores showed acceptable internal consistency (physical α = .75, mental α = .67, self-esteem α = .76, family α = .64, school α = .63), with exception of the subscale friends (α = .54). Therefore, this subscale was excluded from all further analyses. All scores were transformed to standardized 0–100 scores, with higher values corresponding to a subjectively higher QOL. Published reference values for healthy and chronically ill children (Ravens-Sieberer & Bullinger, 2000) were used for further comparisons.
Pain-Related Quality of Life
In addition to the general HRQOL, we assessed the disease-specific HRQOL using the KINDL-illness module. To collect information about abdominal-pain-related QOL, we adapted the illness-module of KINDL-R by replacing “illness” with “abdominal pain” (e.g., “Last week, I was able to manage my abdominal pain quite well”). Internal consistency for pain-related HRQOL in this study was acceptable (α = .68).
Pain-Related Cognitions
The questionnaire for pain-related cognitions (PRCQ-R, Hermann, Hohmeister, Zohsel, Ebinger, & Flor, 2007) is a shortened German version of the Pain Coping Questionnaire (Reid, Gilbert, & McGrath, 1998), which covers 13 items, starting with the phrase “When I’m in pain, …” The PRCQ-R comprises three scales for cognitive coping styles, that is, catastrophizing (e.g., “When I am in pain, I think that the pain never will stop”; 5 items), problem solving (“… , I think about what I could do against the pain.”; 4 items), and positive self-statements (“… , I say to myself: be strong and endure the pain.”; 4 items). Likert-scaling ranges from 1 (never) to 5 (very often). In a validation study with 400 children, the PRCQ-R proved to be internal consistent (Cronbach’s α between .72 and .78), re-test reliable, and valid (catastrophizing showing positive correlations with anxiety, depression, and pain intensity; Hermann et al., 2007). In this study, scale scores showed acceptable (positive self-instruction α = .76; problem-solving α = .76) till good (catastrophizing α = .82) reliability.
Coping Strategies
For assessing behavioral coping strategies, the German version of the Pediatric Pain Coping Inventory (PPCI-revised; Hechler et al., 2008) was applied. Contrary to the English version, only three factors emerged: “positive self-instruction,” “search for social support,” and “passive problem-solving”. The PPCI-revised has demonstrated acceptable internal consistencies (Cronbach’s α between .71 and .80) and has shown to correlate with measures of trait anxiety and pain intensity. We only used the two sub-scales “search for social support” (e.g., “When I am in pain, I tell my mum or my dad.”; 8 items) and “passive problem-solving” (e.g., “… , I lay down.”; 10 items). Likert-scaling is defined as 1 (almost never), 2 (sometimes), and 3 (often), with higher scores indicating a more pronounced use of the particular coping strategy. Internal consistencies in our sample ranged between low (passive problem-solving α = .63) and high (search for social support α = .80).
Pain Symptoms
Children assessed their pain episodes by an adaptation of the Wong–Baker Faces Pain Scale revised (Wong & Baker, 1988). Children were asked to rate the intensity and frequency of their pain during the past 2 weeks (e.g., “In the last 2 weeks, how often did you feel pain?”) on a 6-point Likert scale (0 = no pain till 5 = 5 times or more). As overall measure we defined pain severity (PS) as sum of the ratings for intensity and frequency (range 0–10). Higher scores indicate more severe pain experiences. Reliability of the so-defined PS score was high (α = .80).
Statistical Analysis
All statistical analyses were performed using SPSS 20 for Windows. Before the main analyses, we checked for significant differences between the subsamples in terms of demographic (i.e., age, gender, educational and economic background of the parents) and pain-related variables (i.e., PS, duration since onset) using t-tests or χ2 tests. As shown in Table I, the two groups only differed in terms of PS; therefore, the PS score was included as a covariate. To compare the subsamples in terms of their HRQOL, we performed multivariate analysis of covariance to the scale scores and univariate analysis of covariance to the total score. For examining potential influences of the broad spectrum of diagnoses in the OGID group, all statistical analyses were rerun by excluding children with constipation (n = 10) as well as by differentiating OGIDinflam versus OGIDnoninflam. As this had no effects on the results, only the main comparisons are reported in the following section.
Further, we examined differences in HRQOL for the whole sample as well as for the subgroups FGID and OGID in comparison with German reference values for healthy children as well as for chronically ill children with asthma, atopic dermatitis, and obesity (Ravens-Sieberer & Bullinger, 2000).
Hierarchical multiple regression analysis was performed in search of the best predictor for HRQOL. In the first step, sociodemographic factors (sex, age by median split dividing the sample into groups ≤11 and ≥12 years) were included; in the second step, pain-related variables (PS, duration since onset, diagnosis FGID vs. OGID) were added. In the third step, coping variables (PPCI-revised, PRCQ-R) were included. We checked for presence of multicollinearity by examining tolerance and variance inflation factor. We regarded variance inflation factor values exceeding 10 and tolerance values <.1 as indicating multicollinearity. Mediation analysis was used to examine the relationship between pain, HRQOL, and coping following the procedure described by Baron and Kenny (1986). In all analyses, a p-value <.05 was considered statistically significant. In the case of multiple comparisons by several analyses of variances, significance level was set on α <.001.
Results
Health-Related and Pain-Related Quality of Life
Children with FGID and OGID did not differ in their HRQOL, neither with respect to the subscales [F (5, 162) = 1.13, p = .347] nor with respect to the total score [F (1, 166) = 1.95, p = .165]. No differences were found between the sexes, and HRQOL was not significantly related to age. Further, FGID and OGID groups did not differ in their subjective pain-related QOL [F (1, 166) = .00, p = .993].
Comparison With Normative Scores
We further compared levels of HRQOL across different chronic conditions by using published reference data (Ravens-Sieberer & Bullinger, 2000). As the groups did not differ on HRQOL, the comparative analysis was conducted with the whole sample (CAP; n = 170). Table II displays the results for the comparisons of CAP with healthy children (n = 1,501) and children with asthma (n = 254), atopic dermatitis (n = 163), and obesity (n = 623; Ravens-Sieberer & Bullinger, 2000). Negative values for mean differences imply lower QOL scores for the CAP sample in comparison with the reference samples. The results showed a significantly lower HRQOL for the composite sample on the subscales body, mental, and self-esteem, as well as a significantly lower total score in comparison with healthy children and chronically ill children (asthma and atopic dermatitis). The disease-specific QOL also showed a highly significant difference across groups (Table II). The comparison with the reference sample of children with obesity showed a comparable total score but lower bodily, mental, and disease-specific QOL in the CAP sample. Separate analyses for the subsamples FGID versus OGID yielded the same results.
Comparisons of Mean Values With Data From Reference Samples
| . | . | . | 95% CI for mean difference . | |
|---|---|---|---|---|
| Scale . | Mean difference . | p . | Lower . | Upper . |
| CAP vs. healthy | ||||
| Body | −26.09 | .000* | −29.77 | −22.41 |
| Mental | −14.90 | .000* | −17.94 | −11.86 |
| Self-esteem | −16.13 | .000* | −19.30 | −12.95 |
| Family | −2.79 | .025 | −5.22 | −.36 |
| School | −7.12 | .000* | −10.73 | −3.52 |
| Total score | −12.35 | .000* | −14.45 | −10.23 |
| CAP vs. asthma | ||||
| Body | −21.55 | .000* | −25.23 | −17.87 |
| Mental | −14.24 | .000* | −17.28 | −11.20 |
| Self-esteem | −13.21 | .000* | −16.38 | −10.03 |
| Family | 1.87 | .131 | −.56 | 4.30 |
| School | −1.33 | .466 | −4.94 | 2.27 |
| Total score | −7.28 | .000* | −10.47 | −4.10 |
| Illness | −16.18 | .000* | −19.37 | −12.99 |
| CAP vs. atopic dermatitis | ||||
| Body | −30.47 | .000* | −34.15 | −26.79 |
| Mental | −13.33 | .000* | −16.37 | −10.29 |
| Self-esteem | −12.16 | .000* | −15.33 | −8.98 |
| Family | .44 | .722 | −1.99 | 2.87 |
| School | −2.54 | .165 | −6.15 | 1.06 |
| Total score | −8.31 | .000* | −11.50 | −5.12 |
| Illness | −12.19 | .000* | −15.34 | −9.01 |
| CAP vs. obesity | ||||
| Body | −20.63 | .000* | −24.31 | −16.95 |
| Mental | −11.17 | .000* | −14.21 | −8.13 |
| Self-esteem | −4.24 | .009* | −7.41 | −1.06 |
| Family | 4.42 | .000* | 1.99 | 6.85 |
| School | 3.58 | .052 | −.03 | 7.18 |
| Total score | −2.83 | .081 | −6.01 | .35 |
| Illness | −9.35 | .000* | −12.54 | −6.17 |
| . | . | . | 95% CI for mean difference . | |
|---|---|---|---|---|
| Scale . | Mean difference . | p . | Lower . | Upper . |
| CAP vs. healthy | ||||
| Body | −26.09 | .000* | −29.77 | −22.41 |
| Mental | −14.90 | .000* | −17.94 | −11.86 |
| Self-esteem | −16.13 | .000* | −19.30 | −12.95 |
| Family | −2.79 | .025 | −5.22 | −.36 |
| School | −7.12 | .000* | −10.73 | −3.52 |
| Total score | −12.35 | .000* | −14.45 | −10.23 |
| CAP vs. asthma | ||||
| Body | −21.55 | .000* | −25.23 | −17.87 |
| Mental | −14.24 | .000* | −17.28 | −11.20 |
| Self-esteem | −13.21 | .000* | −16.38 | −10.03 |
| Family | 1.87 | .131 | −.56 | 4.30 |
| School | −1.33 | .466 | −4.94 | 2.27 |
| Total score | −7.28 | .000* | −10.47 | −4.10 |
| Illness | −16.18 | .000* | −19.37 | −12.99 |
| CAP vs. atopic dermatitis | ||||
| Body | −30.47 | .000* | −34.15 | −26.79 |
| Mental | −13.33 | .000* | −16.37 | −10.29 |
| Self-esteem | −12.16 | .000* | −15.33 | −8.98 |
| Family | .44 | .722 | −1.99 | 2.87 |
| School | −2.54 | .165 | −6.15 | 1.06 |
| Total score | −8.31 | .000* | −11.50 | −5.12 |
| Illness | −12.19 | .000* | −15.34 | −9.01 |
| CAP vs. obesity | ||||
| Body | −20.63 | .000* | −24.31 | −16.95 |
| Mental | −11.17 | .000* | −14.21 | −8.13 |
| Self-esteem | −4.24 | .009* | −7.41 | −1.06 |
| Family | 4.42 | .000* | 1.99 | 6.85 |
| School | 3.58 | .052 | −.03 | 7.18 |
| Total score | −2.83 | .081 | −6.01 | .35 |
| Illness | −9.35 | .000* | −12.54 | −6.17 |
Notes. CAP = chronic abdominal pain.
n = 170.
Negative mean difference indicates higher values in the reference samples of healthy or chronically ill children.
Alpha-level corrected (α = .001).
Significant differences marked by *(p < .001).
Comparisons of Mean Values With Data From Reference Samples
| . | . | . | 95% CI for mean difference . | |
|---|---|---|---|---|
| Scale . | Mean difference . | p . | Lower . | Upper . |
| CAP vs. healthy | ||||
| Body | −26.09 | .000* | −29.77 | −22.41 |
| Mental | −14.90 | .000* | −17.94 | −11.86 |
| Self-esteem | −16.13 | .000* | −19.30 | −12.95 |
| Family | −2.79 | .025 | −5.22 | −.36 |
| School | −7.12 | .000* | −10.73 | −3.52 |
| Total score | −12.35 | .000* | −14.45 | −10.23 |
| CAP vs. asthma | ||||
| Body | −21.55 | .000* | −25.23 | −17.87 |
| Mental | −14.24 | .000* | −17.28 | −11.20 |
| Self-esteem | −13.21 | .000* | −16.38 | −10.03 |
| Family | 1.87 | .131 | −.56 | 4.30 |
| School | −1.33 | .466 | −4.94 | 2.27 |
| Total score | −7.28 | .000* | −10.47 | −4.10 |
| Illness | −16.18 | .000* | −19.37 | −12.99 |
| CAP vs. atopic dermatitis | ||||
| Body | −30.47 | .000* | −34.15 | −26.79 |
| Mental | −13.33 | .000* | −16.37 | −10.29 |
| Self-esteem | −12.16 | .000* | −15.33 | −8.98 |
| Family | .44 | .722 | −1.99 | 2.87 |
| School | −2.54 | .165 | −6.15 | 1.06 |
| Total score | −8.31 | .000* | −11.50 | −5.12 |
| Illness | −12.19 | .000* | −15.34 | −9.01 |
| CAP vs. obesity | ||||
| Body | −20.63 | .000* | −24.31 | −16.95 |
| Mental | −11.17 | .000* | −14.21 | −8.13 |
| Self-esteem | −4.24 | .009* | −7.41 | −1.06 |
| Family | 4.42 | .000* | 1.99 | 6.85 |
| School | 3.58 | .052 | −.03 | 7.18 |
| Total score | −2.83 | .081 | −6.01 | .35 |
| Illness | −9.35 | .000* | −12.54 | −6.17 |
| . | . | . | 95% CI for mean difference . | |
|---|---|---|---|---|
| Scale . | Mean difference . | p . | Lower . | Upper . |
| CAP vs. healthy | ||||
| Body | −26.09 | .000* | −29.77 | −22.41 |
| Mental | −14.90 | .000* | −17.94 | −11.86 |
| Self-esteem | −16.13 | .000* | −19.30 | −12.95 |
| Family | −2.79 | .025 | −5.22 | −.36 |
| School | −7.12 | .000* | −10.73 | −3.52 |
| Total score | −12.35 | .000* | −14.45 | −10.23 |
| CAP vs. asthma | ||||
| Body | −21.55 | .000* | −25.23 | −17.87 |
| Mental | −14.24 | .000* | −17.28 | −11.20 |
| Self-esteem | −13.21 | .000* | −16.38 | −10.03 |
| Family | 1.87 | .131 | −.56 | 4.30 |
| School | −1.33 | .466 | −4.94 | 2.27 |
| Total score | −7.28 | .000* | −10.47 | −4.10 |
| Illness | −16.18 | .000* | −19.37 | −12.99 |
| CAP vs. atopic dermatitis | ||||
| Body | −30.47 | .000* | −34.15 | −26.79 |
| Mental | −13.33 | .000* | −16.37 | −10.29 |
| Self-esteem | −12.16 | .000* | −15.33 | −8.98 |
| Family | .44 | .722 | −1.99 | 2.87 |
| School | −2.54 | .165 | −6.15 | 1.06 |
| Total score | −8.31 | .000* | −11.50 | −5.12 |
| Illness | −12.19 | .000* | −15.34 | −9.01 |
| CAP vs. obesity | ||||
| Body | −20.63 | .000* | −24.31 | −16.95 |
| Mental | −11.17 | .000* | −14.21 | −8.13 |
| Self-esteem | −4.24 | .009* | −7.41 | −1.06 |
| Family | 4.42 | .000* | 1.99 | 6.85 |
| School | 3.58 | .052 | −.03 | 7.18 |
| Total score | −2.83 | .081 | −6.01 | .35 |
| Illness | −9.35 | .000* | −12.54 | −6.17 |
Notes. CAP = chronic abdominal pain.
n = 170.
Negative mean difference indicates higher values in the reference samples of healthy or chronically ill children.
Alpha-level corrected (α = .001).
Significant differences marked by *(p < .001).
Predictors of HRQOL
For analyzing predictors of HRQOL, a hierarchical regression analysis was performed. The model significantly explained 11.9% of the variance of HRQOL [F (10, 157) = 3.25, p = .001]. The sociodemographic factors (sex, age) did not explain a significant amount of variance [R2 = .03, F (2, 165) = 2.12, p = .123] in the first step. From the disease-specific variables (PS, duration since onset, diagnosis FGID vs. OGID) in the second step, only the severity of pain symptoms measured by PS explained a significant amount of variance [R2 = .05, F (5, 162) = 2.81, p = .018], whereas diagnosis and duration of disease did not have a significant impact. By looking at the single predictors in the third model, the analysis revealed that catastrophizing was the only coping strategy that significantly predicted children’s HRQOL (β = −.25, p = .006). A higher amount of catastrophizing significantly predicted a lower HRQOL. Table III summarizes the results of hierarchical regression analysis.
Results of Hierarchical Regression Analysis (n = 170)
| . | . | . | . | 95% CI for β . | |
|---|---|---|---|---|---|
| Variable . | β . | t . | p . | Lower . | Upper . |
| Step 1 | |||||
| Gender | .12 | 1.54 | .127 | −.04 | .31 |
| Age | −.10 | −1.36 | .177 | −.06 | .01 |
| Step 2 | |||||
| Gender | .07 | .85 | .399 | −.10 | .26 |
| Age | −.12 | −1.60 | .112 | −.06 | .01 |
| Pain severity | −.23 | −2.88 | .005* | −.09 | −.02 |
| Duration | .05 | .62 | .540 | −.18 | .22 |
| Diagnosis | −.15 | −1.52 | .131 | −.31 | .04 |
| Step 3 | |||||
| Gender | .03 | .40 | .687 | −.14 | .21 |
| Age | −.13 | −1.72 | .087 | −.07 | .00 |
| Pain severity | −.14 | −1.74 | .084 | −.07 | .00 |
| Duration | .03 | .43 | .670 | −.13 | .20 |
| Diagnosis | −.07 | −.96 | .341 | −.26 | .09 |
| Passive problem-solving | −.13 | −1.56 | .120 | −.50 | .06 |
| Search for social support | −.03 | −.35 | .726 | −.26 | .18 |
| Positive self-instruction | .02 | .31 | .761 | −.08 | .11 |
| Problem-solving | .10 | 1.22 | .224 | −.04 | .17 |
| Catastrophizing | −.25 | −2.81 | .006* | −.26 | −.05 |
| . | . | . | . | 95% CI for β . | |
|---|---|---|---|---|---|
| Variable . | β . | t . | p . | Lower . | Upper . |
| Step 1 | |||||
| Gender | .12 | 1.54 | .127 | −.04 | .31 |
| Age | −.10 | −1.36 | .177 | −.06 | .01 |
| Step 2 | |||||
| Gender | .07 | .85 | .399 | −.10 | .26 |
| Age | −.12 | −1.60 | .112 | −.06 | .01 |
| Pain severity | −.23 | −2.88 | .005* | −.09 | −.02 |
| Duration | .05 | .62 | .540 | −.18 | .22 |
| Diagnosis | −.15 | −1.52 | .131 | −.31 | .04 |
| Step 3 | |||||
| Gender | .03 | .40 | .687 | −.14 | .21 |
| Age | −.13 | −1.72 | .087 | −.07 | .00 |
| Pain severity | −.14 | −1.74 | .084 | −.07 | .00 |
| Duration | .03 | .43 | .670 | −.13 | .20 |
| Diagnosis | −.07 | −.96 | .341 | −.26 | .09 |
| Passive problem-solving | −.13 | −1.56 | .120 | −.50 | .06 |
| Search for social support | −.03 | −.35 | .726 | −.26 | .18 |
| Positive self-instruction | .02 | .31 | .761 | −.08 | .11 |
| Problem-solving | .10 | 1.22 | .224 | −.04 | .17 |
| Catastrophizing | −.25 | −2.81 | .006* | −.26 | −.05 |
Note. For step 1 R2 = .013 (ΔF (2, 165) = 2.12, p. = .123); for step 2 ΔR2 = .06 (ΔF (3, 162) = 3.21, p = .025), R2 = .05; for step 3 ΔR2 = .09 (ΔF (5, 157) = 3.48, p = .005), R2 = .12.
Duration = duration since onset of pain in months, coded as <1 year (0) and >1 year (1).
Diagnosis = distinction between FGID and OGID, coded with FGID = 0 and OGID = 1.
*p < .05.
Results of Hierarchical Regression Analysis (n = 170)
| . | . | . | . | 95% CI for β . | |
|---|---|---|---|---|---|
| Variable . | β . | t . | p . | Lower . | Upper . |
| Step 1 | |||||
| Gender | .12 | 1.54 | .127 | −.04 | .31 |
| Age | −.10 | −1.36 | .177 | −.06 | .01 |
| Step 2 | |||||
| Gender | .07 | .85 | .399 | −.10 | .26 |
| Age | −.12 | −1.60 | .112 | −.06 | .01 |
| Pain severity | −.23 | −2.88 | .005* | −.09 | −.02 |
| Duration | .05 | .62 | .540 | −.18 | .22 |
| Diagnosis | −.15 | −1.52 | .131 | −.31 | .04 |
| Step 3 | |||||
| Gender | .03 | .40 | .687 | −.14 | .21 |
| Age | −.13 | −1.72 | .087 | −.07 | .00 |
| Pain severity | −.14 | −1.74 | .084 | −.07 | .00 |
| Duration | .03 | .43 | .670 | −.13 | .20 |
| Diagnosis | −.07 | −.96 | .341 | −.26 | .09 |
| Passive problem-solving | −.13 | −1.56 | .120 | −.50 | .06 |
| Search for social support | −.03 | −.35 | .726 | −.26 | .18 |
| Positive self-instruction | .02 | .31 | .761 | −.08 | .11 |
| Problem-solving | .10 | 1.22 | .224 | −.04 | .17 |
| Catastrophizing | −.25 | −2.81 | .006* | −.26 | −.05 |
| . | . | . | . | 95% CI for β . | |
|---|---|---|---|---|---|
| Variable . | β . | t . | p . | Lower . | Upper . |
| Step 1 | |||||
| Gender | .12 | 1.54 | .127 | −.04 | .31 |
| Age | −.10 | −1.36 | .177 | −.06 | .01 |
| Step 2 | |||||
| Gender | .07 | .85 | .399 | −.10 | .26 |
| Age | −.12 | −1.60 | .112 | −.06 | .01 |
| Pain severity | −.23 | −2.88 | .005* | −.09 | −.02 |
| Duration | .05 | .62 | .540 | −.18 | .22 |
| Diagnosis | −.15 | −1.52 | .131 | −.31 | .04 |
| Step 3 | |||||
| Gender | .03 | .40 | .687 | −.14 | .21 |
| Age | −.13 | −1.72 | .087 | −.07 | .00 |
| Pain severity | −.14 | −1.74 | .084 | −.07 | .00 |
| Duration | .03 | .43 | .670 | −.13 | .20 |
| Diagnosis | −.07 | −.96 | .341 | −.26 | .09 |
| Passive problem-solving | −.13 | −1.56 | .120 | −.50 | .06 |
| Search for social support | −.03 | −.35 | .726 | −.26 | .18 |
| Positive self-instruction | .02 | .31 | .761 | −.08 | .11 |
| Problem-solving | .10 | 1.22 | .224 | −.04 | .17 |
| Catastrophizing | −.25 | −2.81 | .006* | −.26 | −.05 |
Note. For step 1 R2 = .013 (ΔF (2, 165) = 2.12, p. = .123); for step 2 ΔR2 = .06 (ΔF (3, 162) = 3.21, p = .025), R2 = .05; for step 3 ΔR2 = .09 (ΔF (5, 157) = 3.48, p = .005), R2 = .12.
Duration = duration since onset of pain in months, coded as <1 year (0) and >1 year (1).
Diagnosis = distinction between FGID and OGID, coded with FGID = 0 and OGID = 1.
*p < .05.
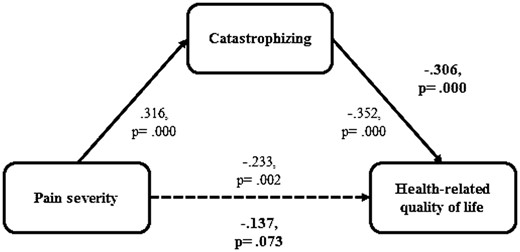
To analyze the relationships found in regression analysis, the significant predictors, PS and catastrophizing, were included in a mediation analysis. As hypothesized, catastrophizing fully mediated the relation between PS and HRQOL. Figure 1 illustrates the relationship for the prediction of children’s HRQOL. Rerunning the analysis for FGID and OGID separately did not change the results.
Mediation analysis. Note: Given are beta-values of preliminary regression analyses and mediation analysis. Bold values indicate results of mediation analysis.
Discussion
The current findings suggest that CAP has a significant effect on HRQOL among children. As hypothesized, children with FGID did not differ in HRQOL from children with OGID, not in relation to the subscales, to the total score, or to the disease-specific module. This confirms and extends the results previously reported on the basis of the Rome II criteria (Varni et al., 2006; Youssef et al., 2006). In contrast to Varni et al. (2007), FGID and OGID did not differ in academic functioning either. However, we included children with IBS in our analyses, who constituted one group of its own besides FGID and OGID in the studies of Varni et al. (2006, 2007). A vast majority of children in the OGID group suffered from food intolerances, which are associated with reduced HRQOL as well (Cummings, Knibb, King, & Lucas, 2010). Overall, the data emphasize that abdominal pain constitutes an increased psychosocial strain for children. The data also provide support for the noncategorical approach (Stein & Jessop, 1982), which states that not the diagnosis per se, rather the general disease characteristics will explain psychological adjustment in chronically ill children.
In addition, HRQOL scores of our sample were compared with data of samples with different chronic diseases. So far, the studies analyzing HRQOL in FGID with other chronic diseases yielded inconsistent results (Gold et al., 2009; Varni et al., 2007). We found significant differences not only in the total score but also in the subscales “body,” “mental health,” “self-esteem,” and in the disease-specific module, pointing to a higher strain experienced by the children with FGID as well as OGID in comparison with those with asthma and atopic dermatitis. This supports the previous research by Gold et al. (2009) pointing out that children suffering from chronic pain report lower HRQOL than those suffering from cancer or rheumatology—both conditions associated with pain experiences themselves. In relation to the subscales “family” and “school,” mainly no differences were found. This may be due to the fact that the items assess mainly the relationship between children and parents and only to a lesser extent, the parental burden often associated with chronic pain (Mano, Khan, Ladwig, & Weisman, 2011). Further studies are necessary to gain differentiated knowledge about the child’s perception of family functioning. The comparison with obese children is particularly interesting. The lower values with regard to bodily, mental, and illness-related QOL of children with FGID and OGID in comparison with obese children and adolescents are astonishing, as obesity is a highly visible disease perceived as controllable by the affected and is therefore associated with a high degree of stigmatization and psychosocial strain (Warschburger, 2005). So far, evidence is unclear concerning the association between FGID, OGID, and obesity, and systematic investigations of this potential relation are lacking.Hainsworth, Davies, Khan, and Weisman (2009) analyzed the influence of weight status on HRQOL in children referred to a pain clinic. Obesity increased the likelihood of an impaired HRQOL by more than twofold (odds ratio = 2.2). In future studies, other groups of chronic diseases should be compared systematically and the influence of comorbid disorders like obesity should be considered more thoroughly in clinical research as well as practice.
In relation to the third hypothesis concerning the prediction of children’s QOL, age and gender proved to be irrelevant, whereas pain intensity and, in the last step, coping style had significant influence, consistent with our hypothesis. This is in accordance with other studies reporting no influence of age and gender on HRQOL (Merlijn et al., 2006), as well as the inverse correlation of pain intensity and HRQOL (Greenley et al., 2013; Varni et al., 2006). Beyond the influence of pain experience, a catastrophizing coping style explained additional 9% of the variance. Thereby, coping style determined the largest proportion of variance in the overall model. Contrary to our expectations, adaptive coping strategies like positive self-instruction or acceptance did not influence HRQOL. Our results are in line with those of van der Zaag-Loonen et al. (2004), who found that coping styles are more influential in explaining the patients’ HRQOL than disease-related variables. These authors report a detrimental effect of a depressive coping style on HRQOL, too.
Our results are extending this finding by revealing that a catastrophizing coping style fully mediates the association between pain experience and HRQOL. Our results support previous studies that stress its essential role in chronic pain research (Huguet et al., 2009). Recently, different models tried to explain the pathways of catastrophizing: (1) the attention model, (2) the interpersonal pain coping model stressing the parent’s role, and (3) the appraisal model stressing the importance of maladaptive beliefs about one’s coping competences (cf. Hermann & Hohmeister, 2012). Cognitive behavioral therapy (CBT) including strategies such as thought stop technique, distraction, relaxation, or positive imaginations has been proven to be successful in the treatment of FGID (Groß & Warschburger, 2013a; Levy et al., 2010; Sprenger, Gerhards, & Goldbeck, 2011). Data from a recent intervention study (Groß & Warschburger, 2013b) show that CBT including thought-control strategies to cope with catastrophizing increases the use of adaptive coping strategies in children suffering from FAP. Derived from the results of this study, those cognitive-behavioral techniques might be helpful for children with OGID as well. Further studies should examine to what extent the reduction of catastrophizing can account for intervention effects.
The strengths of the present study are the systematically analyzed differences and similarities over the groups of children with CAP using a large sample and physician-based diagnosis. In addition, the Rome III criteria were, for the first time, used to explicitly define FGID. The broad age range of the sample, multiple diagnoses, and German-wide recruitment are in favor of generalizability of these current study results. Despite these advantages, we encountered some limitations. First, we used a cross-sectional design to evaluate children’s HRQOL and coping. Therefore, it is strictly speaking not allowed to draw causal conclusions concerning the mediating role of catastrophizing. However, a recent prospective study supports our conclusion: Welkom, Hwang, and Guite (2013) were able to show that the relationship between parental protectiveness and functional disability is mediated by the adolescents’ pain catastrophizing across time. Second, the response rate in our study was low. We cannot rule out that a selection bias occurred. As study inclusion required informed consent, there are no demographic data about noncompleters to examine potential recruitment bias. It is possible that children and parents who were more affected and who used more maladaptive coping strategies were not willing to participate. Just as well it is possible that families with fewer burdens did not feel concerned, or maybe the set of questionnaires was too extensive to deal with while waiting. In addition, the selectivity of a clinical sample restricts general statements. With regard to the definition and composition of the OGID group, the consecutive recruitment strategy yielded unequal representation of diagnoses, with the majority suffering from carbohydrate malabsorption and only 28% showing inflammatory gastrointestinal diseases. Although our sample does reflect quite accurately the population consulting a pediatric gastroenterology, it would be fruitful to include a higher proportion of inflammatory gastrointestinal diseases to analyze group differences with increased statistical power in future studies. Further, most of the instruments showed acceptable to lower reliability, which can restrict the validity of the results. Concerning HRQOL, the use of alternative well-established measures (e.g., PedsQL; Varni, Seid, & Kurtin, 2001; Varni, Kay, Limbers, Franciosi, & Pohl, 2012) should be considered for future studies.
Summing up, the results support the model of psychosocial influences in children suffering from pain. First, the type of illness, that is FGID or OGID, did not discriminate the level of overall HRQOL. Second, the relationship between illness variables and the psychosocial outcome HRQOL was fully mediated by coping style, that is, the higher the level of catastrophizing thoughts, the lower the children’s HRQOL independently of PS. Prospective studies are needed for clarification of effects and interactions.
Acknowledgments
The authors thank all participating children, parents, and clinic staff members for their engagement. Study centers listed by name: Dr. Rüdiger Adam (Mannheim), Dr. Antje Ballauff (Krefeld), Dr. Sebastian Becker (Darmstadt), Dr. Ulrich Gabel (Oberursel), Dr. Ute Kloß (Berlin), Dr. Benno Kretschmar (Eisenach), Dr. Silke Petau (Berlin), Dr. Raymund Pothmann (Hamburg), Prof. Dr. Michael Radke (Potsdam), Dr. Markus Richter (Augsburg), Dr. Oliver Schirrmacher (Vechta), and Dr. Markus Schmitt (Ludwigsfelde).
Conflicts of interest: None declared.