INTRODUCTION
Helicobacter spp. have been isolated from an ever-expanding range of host species[1].
Although gastric non-Helicobacter pylori (H. pylori.) helicobacters (NHPH)[2-5] are ubiquitous in animals, including primates, dogs, cats and pigs, their occurrence in humans is rather low compared to the occurrence of H. pylori. NHPH infection in human gastric mucosa has been suggested to occur through zoonotic transmission[6]. Sequence analysis of 16S rRNA genes detected in NHPH-positive gastric biopsies revealed the presence of 2 sequence types. This has led to the sub-classification of gastric NHPH as “Helicobacter heilmannii (H. heilmannii)” type 1 and “H. heilmannii” type 2. “H. heilmannii” type 1 is identical to Helicobacter suis, which colonizes the stomachs of pigs[7]. “H. heilmannii” type 2 represents a group of species, including Helicobacter felis, Helicobacter bizzozeronii, Helicobacter salomonis, and the previously named “Candidatus H. heilmannii” or “H. heilmannii”[8].
Furthermore, to avoid confusion with the name H. heilmannii, Haesebrouck et al[9] have proposed that H. heilmannii sensu lato (H. heilmannii s.l.) be used to refer to the whole group of non-H. pylori helicobacters detected in the human or animal stomach, for instance, when only histopathology, electron microscopy, or crude taxonomic data are available. The name H. heilmannii sensu stricto (H. heilmannii s.s.) or other species names should be used whenever bacteria are really identified to the species level.
In line with this concept, Smet et al[10] reported the successful isolation of H. heilmannii s.s. (formerly “Candidatus H. heilmannii”) from the gastric mucosa of cats. The bacterium was commonly found in feline rather than canine hosts, and sequence analysis of the urease gene of the bacterium showed high similarity to that of the originally named “Candidatus H. heilmannii”[10,11].
To date, few studies have identified gastric NHPH to the species level by genetic analysis[5,11-14]. Thus far, the differences in the pathogenicity and eradication therapy response between Helicobacter suis (H. suis) and H. heilmannii s.s. infection in humans have not been understood.
We describe here the case of a patient with multiple gastric ulcers infected with H. heilmannii s.s., as defined in a report by Smet et al[10].
CASE REPORT
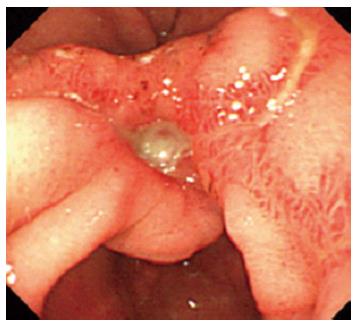
A 62-year-old woman underwent gastrointestinal endoscopy because of the sudden onset of epigastralgia and multiple gastric ulcers were found near the pyloric ring (Figure 1). Gastric tissue biopsies were taken from the ulcer edge and revealed regenerative changes with active inflammation. For the treatment of gastric ulcers, lansoprazole (30 mg/d) was administered once daily to the patient for 4 wk, which resulted in the resolution of her symptoms. Gastrointestinal endoscopic examination after lansoprazole therapy revealed multiple gastric scars near the pyloric ring. Gastric tissue biopsies were taken from the scars and revealed the regenerative changes with active inflammation and the presence of H. heilmannii s.l. on hematoxylin and eosin (H and E) and Giemsa staining in the mucus covering the gastric mucosa; these bacteria were larger and more tightly coiled than H. pylori (Figure 2A). The spiral bacteria were immunostained with a rabbit polyclonal antibody against H. pylori (Dako, Carpinteria, CA) (Figure 2B); this antibody is generated using the whole H. pylori bacterial extract as the antigen, and it exhibits cross-reactivity with “Candidatus H. heilmannii”[15] and H. suis[16]. The cross-reactivity of anti-H. pylori antibodies from different sources with “Candidatus H. heilmannii”[17] and H. suis[18] have been reported to be similar.
Figure 1 Initial gastrointestinal endoscopy shows gastric ulcers in the antrum.
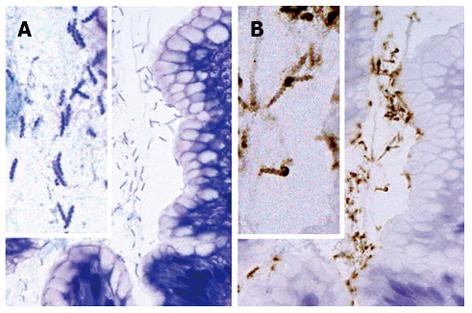
Figure 2 Histological findings in non-Helicobacter pylori helicobacter SH9 in gastric mucosa.
The organisms are present in small groups in gastric pits (A, B). The organisms display a tightly coiled spiral configuration and (B) are positive for the anti-Helicobacter pylori (H. pylori) antibody. (A) HE, original magnification, × 1000; (B) Immunostaining with anti-H. pylori antibody, original magnification, × 1000.
The patient was referred to our hospital for verification of the diagnosis of H. heilmannii s.l.. The patient’s laboratory data were within the normal range. Serum anti-H. pylori immunoglobulin G antibody, measured with an enzyme-linked immunosorbent assay (ELISA) kit using the E plate test (Eiken Kagaku, Inc., Tokyo, Japan), was 2.1 IU/mL (cut-off value: 10 U/mL ). The 13C-urea breath test result (UBiT, Otsuka Pharmaceutical Co., Tokushima, Japan) was negative. The patient was not administered any non-steroidal anti-inflammatory drugs. The patient had 3 household cats.
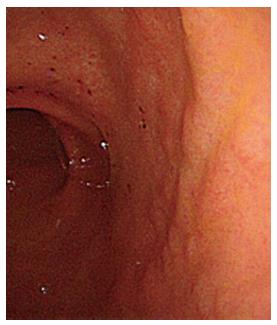
Gastrointestinal endoscopy and mapping gastric biopsy according to the updated Sydney system[19] was performed in our hospital for the histological evaluation of gastritis. In addition, antral biopsy specimens were taken for the culture of H. pylori and for the inoculation of mice by peroral administration of gastric mucosal homogenates. On gastrointestinal endoscopic examination in our hospital, background gastric mucosa of the present patient showed erosive and a slightly coarse appearance in the antrum (Figure 3), and normal appearing mucosa, without atrophy, in the corpus. Histopathological examination of gastric biopsy specimens showed mild mononuclear infiltration with large lymphoid follicles, and without neutrophil infiltration and epithelial degeneration in the antrum (Figure 4) and normal morphology in the corpus. Tightly coiled spiral bacteria were identified in the antrum, but not in the corpus, by histological examination; however, H. pylori was not isolated by microbiological culture. Based on these clinical, endoscopic, and histological findings, a diagnosis of multiple gastric ulcers associated with H. heilmannii s.l. (designated as SH9) infection was made.
Figure 3 Gastrointestinal endoscopy after lansoprazole therapy shows erosion and a slightly coarse granular appearance in the antrum.
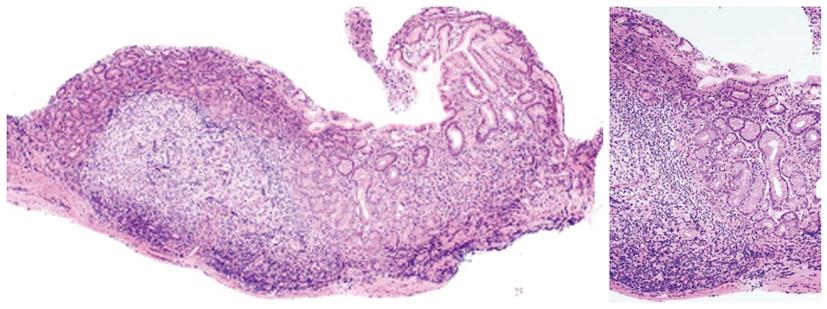
Figure 4 Histological findings of the lesser curvature of gastric antrum from a SH9 (Helicobacter heilmannii sensu stricto)-infected patient after the administration of lansoprazole for the treatment of gastric ulcers.
The gastric mucosa shows large lymphoid follicles with germinal centers without epithelial degeneration.
After confirmation of the H. heilmannii s.l. infection, the patient was treated with 1-wk triple therapy consisting of lansoprazole (30 mg/d), amoxicillin (1500 mg/d), and clarithromycin (1200 mg/d) according to the standard protocol for eradicating H. pylori infection. Twelve wks after the cessation of treatment, gastrointestinal endoscopy showed gastric scars. Further, gastric biopsy mapping showed smaller lymphoid follicles in a biopsy obtained from the greater curvature of the antrum as well as the normal appearance of the corpus, without the presence of these organisms, as evaluated by histology and immunostaining with anti-H. pylori antibody. The patient showed complete remission at the follow-up endoscopy performed 7 years after the completion of eradication therapy, and currently remains healthy.
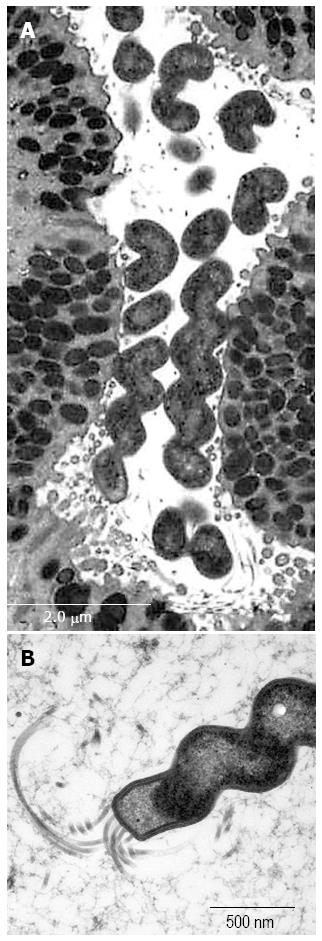
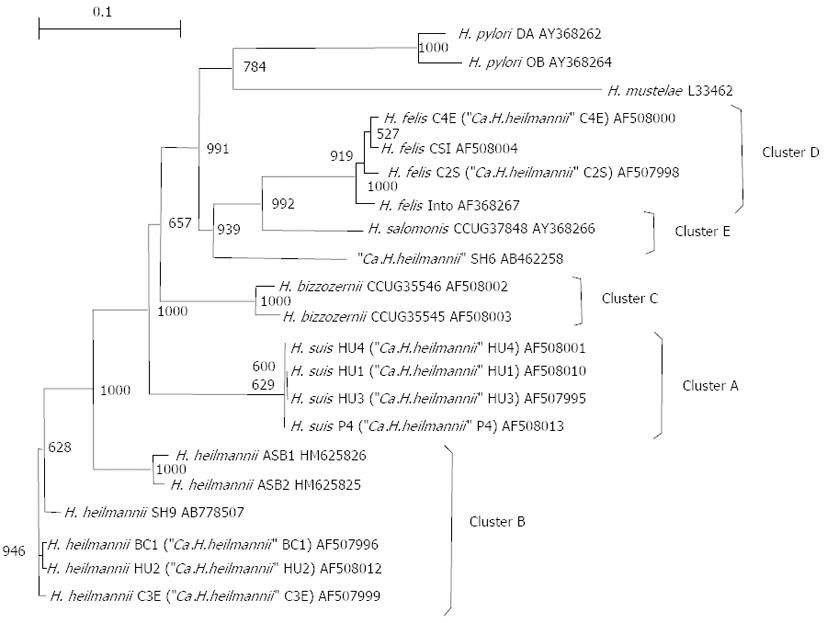
An attempt was made to maintain and propagate SH9 in vivo by inoculating homogenized whole-antral biopsy specimens from the patient into the stomachs of specific pathogen-free mice[20,21]. Transmission electron microscopy of the mouse gastric mucosa colonized with SH9 revealed that the bacteria showed 5-9 coils per cell with flagella at each pole, and were 4-6 μm long and 1 μm wide, which is larger and more coiled than H. pylori (Figure 5). In order to characterize SH9, the 16S rRNA and urease genes were sequenced. Polymerase chain reaction (PCR) amplification of the 16S rRNA gene from SH9-derived DNA isolated from the mouse was performed using 2 sets of primers: 79F (5’-GTGAGTAATGCATAGATGACATGCCC, originally designed in this study) and H679R (5’-ATTCCACCTACCTCTCCCA), and H279F (5’-CTATGACGGGTATCCGGC) and 1494R (5’-TACGGCTACCTTGTTACGAC)[22-24] The urease gene was amplified by PCR using the primers U430F (5’-GCKGAWTTGATGCAAGAAGG)[11] and UH6R2 (5’-TTATCCAAGTGGTGGCACACC)[13] and sequenced. The sequence obtained represented the partial 16S rRNA gene with a readable sequence of 1323 bp (GenBank/EMBL/DDBJ accession no. AB778508) determined from the DNA. The closest match of the 16S rRNA sequence with H. heilmannii s.s BC1 obtained from a bobcat was obtained with 99% (1302/1315 bp) identity (GenBank/EMBL/DDBJ accession no. AF506772). The urease gene from SH9 was amplified by PCR using the primer U430F together with H6-R2. The sequencing represented the partial urease gene with a readable sequence of 1493 bp (GenBank/EMBL/DDBJ accession no. AB778507). The comparatively high similarities of the DNA sequence to that of H. heilmannii s.s HU2 (AF508012) obtained from a human, BC1 (AF507996), ASB2 (HM625825) isolated from an European feline, and ASB1 (HM625826) isolated from an European feline were 98.3% (1467/1493 bp), 98.3% (1427/1451 bp), 90.5% (964/1065 bp), and 90.4% (958/1060 bp), respectively. The DNA sequences of the urease genes from SH9 and from reference organisms were aligned using GENETYX ver. 9 (GENETYX Corp., Tokyo, Japan), and the resulting alignment was modified to remove regions containing gaps. A phylogenetic tree was constructed according to the neighbor-joining method[25,26] and the Kimura model. The stabilities of the relationships were assessed by bootstrap analysis comprising 1000 data settings. Figure 6 demonstrates a phylogenetic tree constructed on the basis of urease gene analysis, and grouping of clusters as shown by O’Rourke et al[11]. The results demonstrated high levels of homology with cluster B.
Figure 5 Ultrastructure of SH9 (Helicobacter heilmannii s.
s.) infected mouse gastric mucosa. The organisms showing tightly coiled spiral configuration (A) with flagella (B) are positioned on the apical surface of the surface mucous cells.
Figure 6 Phylogenetic tree based on partial ureA and ureB gene sequences, demonstrating the relationship between Helicobacter spp.
from animals and humans. Clusters A through E correspond to Helicobacter suis (H.suis), Helicobacter heilmannii sensu stricto (H. heilmannii s.s.), Helicobacter bizzozerronii (H. bizzozerronii), Helicobacter felis (H. felis), and Helicobacter salomonis (H. salomonis), as described by O'Rourke et al[11]. Ca.H.heilmannii: Candidatus H. heilmannii; H. pylori: Helicobacter pylori.
DISCUSSION
We investigated the H. heilmannii s.s., designated as SH9, of human origin, both histologically and genetically by analysis of the sequences of both the 16S rRNA and urease genes.
We were unable to detect H. pylori, but detected a population of corkscrew-shaped organisms in gastric tissue biopsy samples taken from the marginal region of the gastric ulcers and from the antrum. The organisms were found in the mucous gel or on the surface of mucous cells without adhesion to gastric epithelial cells. Background gastric mucosa of the patient showed chronic gastritis with prominent lymphoid follicles without atrophy, although the mapping biopsy was performed after administration of lansoprazole for the treatment of gastric ulcers. These findings are similar to those obtained in previous studies of H. heilmannii s.l.[15,27-29]. The negative antibody test and urea breath-test results of the present patient were also consistent with previous results of H. heilmannii s.l. infection[15]. The sensitivity of urea breath-test for detection of H. heilmannii s.l. was reported to be low[30] and in this study, urea breath-test was negative. A false-negative urea breath-test test may occur due to the lower activity of H. heilmannii s.l. urease, as suggested by previous studies[30-32].
The 16S rRNA gene sequence of SH9 exhibited 99.1% (1302/1314 bp) similarity to H. heilmannii s.s. BC1; this was higher than that to H. suis. In the subsequent comparison of the urease gene sequence, SH9 revealed apparently higher levels of urease gene sequence similarities to H. heilmannii s.s. HU2 and H. heilmannii s.s. BC1. In the phylogenetic analysis following sequencing of the urease genes, SH9 was classified as belonging to Cluster B, proposed by O’Rourke et al[11] (Figure 6). Consequently, we identified SH9 as H. heilmannii s.s.. Interestingly, homologies to the urease gene sequences of H. heilmannii s.s. ASB1 and ASB2 isolated from European felines were approximately 90%. The slightly differences between the urease genes of SH9 and the European isolates may suggest geographical differences.
H. heilmannii s.l. infection have been reported in patients with acute[29] and chronic gastritis[15,27,28], duodenal ulcers[33], gastric mucosa-associated lymphoid tissue (MALT) lymphoma[15,27,34], or gastric carcinoma[35]. However, since identification at the species level of H. heilmannii s.l. is often lacking, the pathogenicity of H. suis and H. heilmannii s.s. has not been clear. H. suis infection has, however, been reported to cause gastric MALT lymphoma in experimental animals[16,18,36]. In contrast to H. suis, H. heilmannii s.s. infection in humans has rarely been reported. Interestingly, Dieterich et al[12] reported multiple H. heilmannii s.l. strains infection in a gastric ulcer patient and in his 2 cats after partially analyzing the urease B gene and suggested transmission of H. heilmannii s.l. from the patient’s household cats. Based on partial urease B gene sequences of H. heilmannii s.l. strains in their report, all of their H. heilmannii s.l. strains can be defined as H. heilmannii s.s. as the partial urease B gene sequences they reported demonstrated the highest similarity 98% with H. heilmannii s.s (GenBank/EMBL/DDBJ accession no. L25079). In addition, the reported H. heilmannii s.s. isolates have commonly been observed in feline rather than canine hosts[11]. The present patient also had 3 cats, again suggesting transmission of H. heilmannii s.s from the patient’s household cats.
H. heilmannii s.l. eradication by antimicrobial treatment regimens that are used for eradicating H. pylori results in clinical and histological improvement in H. heilmannii s.l.-associated gastroduodenal diseases[15,29,33,34,37]. Furthermore, in the present case, 1-wk triple therapy (amoxicillin, clarithromycin, and lansoprazole) for the eradication of H. pylori was effective. Thus, the regimen for H. pylori eradication may also be effective for H. heilmannii s.s. PCR analysis is desirable for the precise evaluation of H. heilmannii s.s infection status after eradication therapy. In the present case, H. heilmannii s.s. was considered to be successfully eradicated, because the patient showed complete remission at the follow-up endoscopy performed 7 years after the completion of eradication therapy. Currently, the patient is healthy.
In conclusion, we presented a case of H. heilmannii s.s.-associated multiple gastric ulcers. Further studies are needed to clarify the pathogenicity of H. heilmannii s.s. and to verify effective eradication strategies for H. heilmannii s.s..
COMMENTS
Case characteristics
A 62-year-old female with Helicobacter heilmannii sensu stricto (H. heilmannii s.s.)-related gastric ulcers.
Clinical diagnosis
Sudden onset of epigastralgia.
Differential diagnosis
Gastroesophageal reflux disease, Gastric cancer, nonsteroidal anti-inflammatory drug-related gastric uler, Helicobacter pylori (H. pylori)-related gastric ulcer.
Laboratory diagnosis
Laboratory data were within the normal range. Serum anti-H. pylori immunoglobulin G antibody and the 13C-urea breath test result were negative.
Imaging diagnosis
Gastroscopic examination revealed multiple gastric ulcers near the pyloric ring.
Pathological diagnosis
Pathological and molecular biological examinations revealed the gastric ulcer with H. heilmannii s.s. infection.
Treatment
The patient was treated with 1-wk triple therapy consisting of lansoprazole (30 mg/d), amoxicillin (1500 mg/d), and clarithromycin (1200 mg/d) according to the standard protocol for eradicating H. pylori infection.
Related reports
H. heilmannii s.s. infection in humans is not very well reported.
Term explanation
The name Helicobacter heilmannii sensu stricto is H. heilmannii identified to the species level.
Experiences and lessons
H. heilmannii s.s. is also related to a human gastric ulcer.
Peer review
This article shows a rare case of H. heilmannii s.s. infection in a patient with multiple gastric ulcers.
P- Reviewers: Daphan CE, Ulasoglu C S- Editor: Wen LL L- Editor: A E- Editor: Wu HL