Article Text
Abstract
Background Many children and adolescents with juvenile idiopathic arthritis experience lower limb problems which may lead to physical disabilities significantly impacting on their quality of life and symptoms. Emerging evidence has identified the effective role of podiatry in the management of juvenile idiopathic arthritis, suggesting the clinical benefit of different orthotic therapies.
Methods This study will be a parallel-group designed, multicentre, randomised controlled trial, aiming to recruit 66 children and adolescents with juvenile idiopathic arthritis aged between 5 and 18 years. Those recruited will need to be diagnosed according to the International League of Associations for Rheumatology criteria, and present with lower limb joint pain, swelling and/or tenderness. Participants will be recruited from three outpatient hospital clinics in New South Wales, Australia. Participants will be randomly allocated to receive a trial or control intervention. The trial group will be prescribed a customised preformed foot orthoses; instead, the control group will receive a flat 1 mm insole with no corrective modifications. Primary outcome measure recorded will be pain. Secondary outcomes will be quality of life, foot disability, swollen and tender joint count and gait parameters (such as plantar pressures, walking speed, stance and swing time). The allocated foot orthoses will be worn for 12 months, with data collected at baseline, 4 weeks, 3, 6 and 12 months intervals. Group allocation will be concealed and all analyses will be carried out on an intention to treat.
Discussion The purpose of this trial is to explore the efficacy of a cost-effective, non-invasive podiatric intervention that will be prescribed at the initial biomechanical consultation. This approach will promote early clinical intervention, which is the gold standard in paediatric rheumatology. Furthermore, this study has the potential to provide new evidence for the effectiveness of a mechanical intervention alone to reduce swollen and tender joints in juvenile idiopathic arthritis.
Trial registration number This clinical trial has been registered with the Australian New Zealand Clinical Trials Registry: ACTRN12616001082493p. Ethics for this randomised controlled trial has been approved (16/09/21/4.03).
- rheumatology
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Statistics from Altmetric.com
What is known about the subject?
Custom or customised foot orthoses may reduce lower limb pain and improve quality of life in children with juvenile idiopathic arthritis. No research has explored the effectiveness of a mechanical intervention alone for reducing swollen and tender lower limb joints in juvenile idiopathic arthritis.
What this study hopes to add?
This study may inform clinical practice on the benefit of prescribing a customised preformed foot orthoses as part of a multidisciplinary approach, in the management of lower limb problems in juvenile idiopathic arthritis.
Introduction
Juvenile idiopathic arthritis (JIA) is the most common chronic rheumatic disease that affects children and adolescent and may cause short-term and long-term disability.1 Prevalence estimated in high-income countries range from 16 to 400 per 1 00 000.1 2 The lower limb appears to be more commonly involved in JIA, particularly the oligoarticular subtype.1 3 The manifestation of JIA includes joint swelling, effusion, tenderness and painful limitation with joint movement, discrepancies in limb development and fatigue.1 4 Lower limb impairments may reduce physical activities compared with healthy children.5–7 As a result, this may account for the lower aerobic capacity among children with JIA.8 Initially, the main aim for the multidisciplinary team should be to relieve pain and discomfort, to reduce joint inflammation, promote function and prevent deformities. However, the provision of healthcare to children with JIA has been reported to be particularly challenging,9–12 with podiatric care currently very limited within paediatric rheumatology in Australian hospitals.
Few studies have evaluated the effectiveness of a custom or customised preformed foot orthoses (FOs) in children with JIA.13 14 A meta-analysis of the effectiveness of a custom or customised FOs in children with JIA indicated that FOs may hold clinical importance in outcomes such as pain and quality of life; however, between-group differences in means were predominately insignificant with imprecise and broad CIs.15 Moreover, a randomised controlled trial investigating the effectiveness of a multidisciplinary foot care programme found no statistical significant difference between groups.16 The programme included a multifaceted approach to foot care in JIA; including ultrasound-guided intracorticosteroid injections, paediatric rheumatology, podiatry and physiotherapy.16 The authors reported challenges in participant recruitment and retention; leading to an underpowered study which may have been vulnerable to type II error.16 Until more research is conducted, the effectiveness of FOs in children with JIA remains to be further explored.15 A recent study in patients with early rheumatoid arthritis suggests that by correcting biomechanical misalignments and reducing mechanical trauma of foot and ankle joints using a customised preformed FOs, it may result in a reduction of swelling and joint tenderness.17 The effectiveness of a customised preformed FOs alone for the reduction of lower limb swollen and tender joints in JIA has never been investigated. This randomised controlled trial aims to provide new evidence on the effectiveness of a non-invasive, mechanical therapy for the reduction of swollen and tender joint counts in children with JIA. It will also further explore the effectiveness of customised preformed FOs in reducing lower limb pain, improving quality of life and gait parameters in children and adolescents affected by this rheumatic disease.
Objectives
The objective of this study is to investigate the effectiveness of a customised preformed FOs in reducing lower limb pain, swollen and tender joints and in improving quality of life and gait in children with JIA.
Hypotheses
Primary outcomes
Preformed FOs will reduce lower limb pain using visual analogue scale (VAS) in children with JIA.
Secondary outcomes
Preformed FOs will improve quality of life in children with JIA using the Pediatric Quality of Life Questionnaire (PedsQL) Rheumatology Module—V.3.0 for children and parents.
Preformed FOs will improve foot disability in children with JIA using the Juvenile Arthritis Foot Disability Index (JAFI).
Preformed FOs will reduce localised swelling and tenderness of lower limb joints in children with JIA by visual inspection and palpation.
Preformed FOs will have an effect on quantitative kinematic and kinetic parameters of gait in children with JIA when barefoot, with shoes alone, and with shoes and FOs.
Trial design
To test the hypothesis, this study will be a parallel-group designed, multicentre, randomised controlled trial. The allocation ratio of participants to their groups will be 1:1.
Methodology
Study setting
This randomised controlled trial will be conducted across three outpatient paediatric rheumatology clinics in New South Wales, Australia: John Hunter Children’s Hospital (Newcastle); Sydney Children’s Hospital Network (Randwick and Westmead).
Eligibility criteria
The inclusion and exclusion criteria are as follows:
Inclusion criteria
Diagnosed with JIA according to the International League of Associations for Rheumatology criteria.
Aged 5–18 years.
Active involvement of the lower limb (including foot/ankle and/or hip and knee involvement).
No previous use of FOs, or previous failure of foot orthotic management, where the patient has not worn any FOs for a period of at least 3 months.
If disease-modifying antirheumatic drugs and/or biological therapy are used, not having started these drug therapies within 6 months of enrolling in the trial.
Exclusion criteria
Inability to walk barefoot or shod for 15 m without assistive devices.
Concomitant musculoskeletal disease, central or peripheral nerve disease and endocrine disorders, including diabetes mellitus.
History of lower limb surgery that required general anaesthetic.
Currently using FOs.
Where prescription of FOs is contraindicated, for example, significant osseous abnormalities noted in the lower limbs and/or vertebrae during the physical evaluation. Unwillingness to wear appropriate footwear for fitting orthoses.
Control group
Participants in the control group will receive a flat insole without any corrective modifications. The control insole will be made with 1 mm thick leather board, top covered by a ‘Dual Opulex Performance’ 1.5 mm thick material made from a neoprene base and a stretch nylon top. The top cover is representative of standard insole appearance regularly supplied by podiatrists.
Trial group
The trial group will receive a customised, pre-formed FOs with the same ‘Dual Opulex Performance’ top cover. The prescription of the FOs (SlimFlex Simple, Algeos) will be customised at chair side according to each individual biomechanical need, and supplied on the same day of the initial assessment. Supplying the FOs on the same day of the initial assessment promotes early clinical intervention, which is the gold standard approach in JIA.1 18 Chief investigator (AF) will issue the trial and control orthoses to participants.
Footwear
Participants in both the control and trial group will receive footwear education and recommendations. Footwear advice will focus on suitable cushioning properties and supportive features of a shoe, appropriate sizes and details related to orthoses fitting. Information will be conveyed in a manner that is easy to understand for both participants and their parents.
Usual care
Throughout the duration of the trial, participants in both the control and trial groups will continue their usual outpatient care at the John Hunter Children’s Hospital and Sydney Children’s Hospital Network. Outpatient care may include but not limited to a paediatric rheumatologist, specialist nurse, physiotherapist, occupational therapist, psychologist, etc.
Compliance/adherence
Adherence to the control and trial intervention will be monitored with visual inspection of FOs at follow-up consultations. Participants will be asked to fill a self-reported diary that will have to be completed for the duration of the trial in order to record the overall number of days per week they have worn their FOs. Participants will be required to bring their diary to their next follow-up consultation and will be given a new one to complete and return. Chief investigator (AF) will provide verbal and written instructions to both participants and parent on how to correctly wear the FOs, as well as clinical support if any problems arise. In case, patient’s foot size will increase during the data collection period and become unsuitable, the exact same prescription will be replicated in a new longer device and supplied to the participant.
Pharmaceutical changes will be recorded over the 12-month period. If changes occur to participant’s medications, it will be noted and patients will be classified as ‘medication changed’. This approach is fundamental to account for any positive effect to be solely attributed to the FOs intervention and not to the medication changes.
Outcomes
Primary outcomes
Pain
Participants in both groups will be asked to rate their lower limb pain on a 100 mm VAS. Pain will be self-reported by participants and their parents/carer. A clinical important difference is considered when there is an 8 mm difference between time intervals.19 When measuring pain with the 100 mm VAS, a lower score indicates less pain and therefore is a better outcome. Pain scores will be collected at baseline, 4 weeks, 3, 6 and 12 months follow-up consultations, with 12 months representing the end point for primary outcome assessment.
Secondary outcomes
Quality of life
Quality of life will be measured using the PedsQL. The PedsQL (module 3) is a self-reported questionnaire, which will be independently supplied to both the participants and parents/carer.20 The PedsQL V.3.0 Rheumatology Module is specific for children with JIA by measuring different outcome subheadings such as: ‘pain and hurt’, ‘daily activities’, ‘treatment’, ‘worry’ and ‘communication’.20 A clinical important difference is considered when there is a 5-point difference between time intervals.20 The PedsQL questionnaire is scored out of 100 points, and a higher score is indicative of a better quality of life. The PedsQL is available in different age ranges: 5–7; 8–12 and 13–18. Quality of life is self-assessed and will be measured at baseline and 3, 6 and 12 months time point.
Foot disability
Foot disability will be measured using the JAFI, which comprises 3 components (27 responses in total): foot and ankle impairment, activity limitation and participation restriction.21 The JAFI has shown to be a reliable and valid measure of foot-related disability in children with JIA.21 Foot disability using the JAFI will be measured at baseline and 3, 6 and 12 months time.
Swollen and tender joint
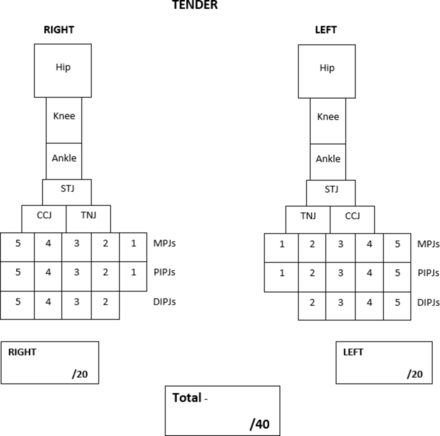
Helliwell et al 22 developed a tool to record swollen and tender foot and ankle joints specifically for adult rheumatoid arthritis.22 This tool was used in an earlier podiatric-based study in rheumatoid arthritis17; and with some further modifications (adding hip, knee, distal and proximal interphalangeal joints), will be used to assess lower limb joint swelling and tenderness count for this randomised controlled trial. Joint count for swelling and tenderness will be recorded at baseline and 6-months follow-up assessments. The assessment tools for swelling and tenderness are depicted in figures 1 and 2, respectively. Physical examination will be carried out by paediatric rheumatologists DSG/JC who will be blinded to the participant’s treatment allocation.
Swollen lower limb joint count.
Tender lower limb joint count.
Gait parameters
Gait analysis will be completed at baseline, 3 and 6 months. In-shoe and plantar pressure analysis will be carried out by using the latest Wireless F-Scan and HR Mat (Tekscan, Boston, USA), respectively. Both systems are equipped with the same sensors resolution allowing for calibration and data analysis comparison. Barefoot, shod and shoes with the FOs recordings sequence will be randomised to account for fatigue potentially exhibited by children with symptomatic JIA during acquisition of gait data. Outcome measures for gait parameters include:
Peak pressure and pressure time integral data will be extrapolated from the following anatomical areas: total contact; heel; midfoot; rearfoot; first metatarsal head; second metatarsal head; third/fourth metatarsal head; fifth metatarsal head; lesser digits; distal phalanx of hallux.
Walking speed (m/s)
Stance time (s)
Swing time (s)
Double limb support (s)
Stride length (cm)
Biomechanical assessment
Standardised assessments such as the Foot Posture Index will be conducted to clinically assess the lower limb, and inform the prescription and modifications of foot orthoses. Muscle bulk and limb length discrepancies will be observed and recorded as part of the Paediatric Gait Arms Legs Spine (pGALS) Legs assessment. The following additional biomechanical and anatomical test will be carried out: range of motion of lower limb joints; gait analysis; presence of enthesitis (Achilles and plantar fascia) and presence of tendonitis (eg, tibialis posterior, peroneal and Achilles tendons).
Timeline of participants
Participants will only be required to attend three data collection sessions: baseline, 3 and 6 months (4-week and 12-month follow-ups are self-assessed only); and it is expected that each meeting will last no more than 1 hour. Participants, in both groups, will be supplied their allocated FOs at the same day of baseline assessment and will be required to wear their FOs for 12 months. Figure 3 depicts a schematic diagram of the participant timeline.
Participant timeline. FOs, foot orthoses.
Sample size
Currently, there are insufficient data to conduct power calculations for each outcome. However, sufficient data are available with regard to pain outcome. Power calculations are therefore based on the outcome of pain measured on a 100 mm VAS, with a minimal clinical significance of 8 mm.19 For a two-sided t-test with α=0.05 and power 80% for a randomised controlled trial design with baseline and primary outcome of difference between the groups at 12 months, and a moderate effect size of 0.6, it was estimated that a total of 90 participants would be required (45 controls and 45 trial) on a ratio of 1:1 allocation.23 24 This was adjusted with an analysis of covariance using an assumed correlation of 0.6 giving an adjusted total number of participants required of 60 (30 controls and 30 trial). The study will be overpowered to an estimated 66 (ie, 33 participants per group) to allow for 10% dropouts during the 12-month data collection period.
Recruitment
Participants will be recruited from the outpatient paediatric rheumatology clinics listed in the study setting section. Parents and patient will be asked if they would like to be part of the study when attending their scheduled consultation with the paediatric rheumatologist. If the patients are eligible and their parents/carers are interested in being involved they will then be referred to the chief investigator (AF).
Allocation
Sequence generation
Immediately after consent is obtained, participants will be randomly allocated in blocks of 10, to either a control or trial group. Their allocation to each group will be achieved with a predetermined randomly generated list. This will be achieved using a computer random number generator (https://www.random.org/sequences/).
Allocation concealment
Allocation concealment will be achieved by using sequentially numbered, opaque and sealed envelopes. Both sequence generation and allocation concealment will be conducted by a research team member. During this independent allocation process, the research team member will not be involved at any stage as part of the recruitment process, orthotic prescription of the control or trial intervention, data collection and will not have any prior or ongoing contact with enrolled participants.
Blinding
Participants and their carer’s in both groups will be blinded to what intervention they receive. The external aesthetic appearance of both the control and trial FOs will be the same by using the ‘Dual Opulex Performance’ top cover. To reduce bias during the data analysis stage, once data collection is complete, the participants' and parents‘/carers’ identity and their intervention will be coded and deidentified. Outcome assessors will be blinded to all self-reported outcomes such as pain (VAS), quality of life (PedsQL) and foot disability (JAFI).
Data analysis
All data collected during the study from baseline, 4 weeks, 3, 6 and 12 months will be represented graphically using the histograms and/or box plots. Normality will be checked using the Shapiro-Wilk test. Where the variable presents ordinal data, the appropriate non-parametric test will be used. A series of between-group and within-group analyses will be carried out. Analyses will be conducted with intention to treat.
Comparison between control and trial group
To test the hypothesis, the control group will be compared with the trial group. Pair-wise statistical analysis will be carried out using an unpaired t-test or Mann-Whitney U test, depending on the distribution of the data.
Within each individual group analysis
To investigate the relationships between the groups at baseline, 4-weeks, 3, 6 and 12 months, a repeated measures analysis of variance will be carried out for parametric data where sphericity exist; or a Friedman test where non-parametric and/or no-sphericity exists.
Limitations
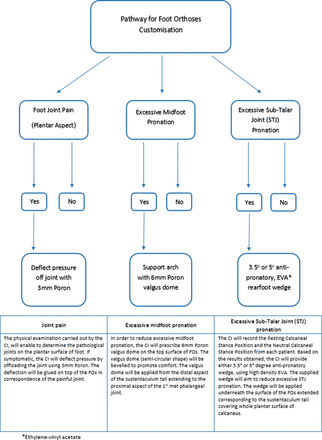
Few limitations should be noted as part of this protocol. Joint disease in JIA may be subclinical and therefore may not be detected using physical examination alone. The use of a standardised lower limb physical examination tool may improve the validity of findings in the swollen and tender joint outcome. Lastly, the orthoses intervention will vary based on the individual biomechanical needs of each participants and therefore may differ. In order to enhance the reproducibility of the trial intervention, each FO modifications will be standardised using a clinical decision pathway (figure 4).
Pathway for FOs customisation.
Data management
All electronic data will be kept on a University approved and prescribed laptop that will be password protected. The data will also be stored on a backup external hard drive that will be under lock and key in a metal cabinet on the University premises. The information from the research project will be stored for a period of 5 years after the completion of the research project, in accordance with the University of Newcastle’s ‘stored data policy S000922’.
References
Footnotes
Contributors AF, DS-G, DS, JC and AC were actively involved in the conception and design of the study. AF and AC drafted the protocol, and DS-G, DS and JC revised the draft and critically appraised for intellectual content. Finally, all authors read and approved the final manuscript prior to submitting for publication.
Funding University of Newcastle—part of a PhD scholarship.
Competing interests None declared.
Patient consent Obtained
Ethics approval Hunter New England Human Research Ethics Committee. Site authorisation has also been approved for all data collection sites by the relevant research governance committees. Ethical considerations: participants can withdraw from the trial at any stage without providing any reason. If participants in the trial group benefited from the FOs and their symptoms improved, they will be informed to keep them and given referral information to seek out further podiatric care if needed. If the trial intervention is found to be beneficial and data analysis confirms this, participants in the control group will be given the option of a free consultation and prescription of the trial FOs used in the study.
Provenance and peer review Not commissioned; externally peer reviewed.