Article Text
Abstract
Objectives To study (1) epidemiological factors, clinical profile and outcomes of COVID-19 related multisystem inflammatory syndrome in children (MIS-C), (2) clinical profile across age groups, (3) medium-term outcomes and (4) parameters associated with disease severity.
Design Hospital-based prospective cohort study.
Setting Two tertiary care centres in Kerala, India.
Participants Diagnosed patients of MIS-C using the case definition of Centres for Disease Control and Prevention.
Statistical analysis Pearson χ2 test or Fisher’s exact test was used to compare the categorical variables and independent sample t-test or Mann-Whitney test was used to compare the continuous variables between the subgroups categorised by the requirement of mechanical ventilation. Bonferroni’s correction was used for multiple comparisons.
Results We report 41 patients with MIS-C, mean age was 6.2 (4.0) years, and 33 (80%) were previously healthy. Echocardiogram was abnormal in 23 (56%), and coronary abnormalities were noted in 15 (37%) patients. Immunomodulatory therapy was administered to 39 (95%), steroids and IVIg both were used in 35 (85%) and only steroids in 3 (7%) patients. Intensive care was required in 36 (88%), mechanical ventilation in 8 (20%), inotropic support in 21 (51%), and 2 (5%) patients died. Mechanical ventilation requirement in MIS-C was associated with hyperferritinaemia (p=0.001). Thirty-seven patients completed 3 months follow-up by April 2021, of whom 6 (16%) patients had some residual echocardiographic changes.
Conclusions Patients with MIS-C in our cohort had varied clinical manifestations ranging from fever with mild gastrointestinal and mucocutaneous involvement to fatal multiorgan dysfunction. Immediate and medium-term outcomes remain largely excellent except for the echocardiographic sequelae in a few patients which are also showing a resolving trend. Hyperferritinaemia was associated with the requirement of mechanical ventilation.
- COVID-19
Data availability statement
Data are available upon reasonable request. Please contact sumabalan@gmail.com for any request related to data sharing.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
What is known about the subject?
Multisystem inflammatory syndrome in children (MIS-C) is a rare but critical association of COVID-19 infection in children.
MIS-C is known to present as a hyperinflammatory state with fever, gastrointestinal, mucocutaneous symptoms, atypical Kawasaki disease-like phenotype and macrophage activation syndrome.
What this study adds?
In our cohort of MIS-C, patients presented at a younger age with more frequent mucocutaneous changes and lesser comorbidities as compared with western studies.
The medium-term outcome of patients with MIS-C is excellent; however, we need to monitor echocardiogram at subsequent follow-up visits in selected patients.
We were able to associate hyperferritinaemia with requirement of mechanical ventilation in patients with MIS-C.
Introduction
The pandemic of SARS-CoV-2 is rapidly evolving. As of 13 August 2021, there have been 205 338 159 confirmed cases of COVID-19 globally, including 4 333 094 deaths.1 Earlier studies reported that COVID-19 infection in children was either asymptomatic or mild with only a small proportion requiring hospitalisation and lesser mortality as compared with adults.2
In May 2020, several European countries reported clusters of hyperinflammatory processes in children with clinical manifestations of atypical Kawasaki disease (KD) and shock and the possibility of its link with SARS-CoV-2 was considered.3–6 Later, the Centers for Disease Control and Prevention (CDC) and WHO released health advisories and defined these cases as multisystem inflammatory syndrome in children (MIS-C) associated with COVID-19.7 8 MIS-C is a rare but severe and potentially fatal condition.9 10
The pathogenesis of MIS-C is not well understood. It is known that SARS-CoV-2 enters cells by binding to ACE 2, which is highly expressed in cardiac myocytes, alveolar cells,vascular endothelium and a small subset of immune cells.11 Evidences suggest that dysregulated innate immune response leading to cytokine storm and endothelial damage might be responsible for multiorgan failure in severe COVID-19 and MIS-C.12–14
MIS-C is reported to present as a hyperinflammatory state with fever, gastrointestinal (GI), mucocutaneous symptoms, atypical KD-like phenotype and macrophage activation syndrome. It is a syndromic presentation with overlapping clinical features of KD, sepsis, toxic shock syndrome and meningitis.9 14 Moreover, there is no diagnostic test and the risk factors for the development of MIS-C remain unknown.
There is a paucity of data from the Indian subcontinent regarding the clinical course of MIS-C. In this study, we have described clinical profile, medium-term outcomes, varied clinical features in different age groups, and factors associated with severe illness in 41 patients diagnosed with MIS-C from southern Indian state of Kerala.
Methods
Study design
This was a hospital-based prospective cohort study conducted at two tertiary care centres from the Kerala state of India, from March 2020 to April 2021. The primary objective of this study was to report the baseline characteristics, clinical features, laboratory parameters, echocardiographic findings, treatment and immediate outcomes of cases admitted with MIS-C. The secondary objectives were (1) to study the clinical presentation and response to therapy across age groups, (2) to report the medium-term outcomes of MIS-C, and (3) to report the predictors of severity in MIS-C.
Patient and public involvement
The study was approved by the institutional ethics committee—Institutional Review Board of Amrita Institute of Medical Sciences (IRB-AIMS-2020–335) which involved public representatives as well. A written informed consent was obtained from the parents of study participants. Patients were not involved in the designing of the study.
Study definitions
We used the CDC case definition to define a case of MIS-C.7 Body mass index (BMI)-based overweight and obesity were defined using Indian standard reference for BMI and it was calculated in age groups comprising of patients more than 5 years of age.15 For the cases under 5 years of age, overweight was defined as weight-for-height greater than 2 SD above WHO child growth standards median; and obesity was defined as weight-for-height greater than 3 SD above the WHO child growth standards median.16
Systolic dysfunction was defined by reduced left ventricular ejection fraction (LVEF). Systolic dysfunction was categorised as mild to moderate when LVEF was 30%–55% and as severe if LVEF was less than 30%.17 18
Echocardiography Z-scores were calculated using Mc Crindle et al19 formula using body surface area. Coronary artery abnormalities (CAA) were classified according to the Z-scores on echocardiography.20 Echocardiographic appearance of hyperechogenicity and non-tapering morphology were also noted as abnormalities.21
For this study, ‘Incomplete KD’ was defined as the presence of fever with less than four out of the five principal clinical criteria with compatible laboratory or echocardiography findings.22 Children who along with the usual clinical features of KD also had few unusual clinical manifestations like pulmonary involvement and renal impairment were labelled ‘atypical KD’.22
Categorisation of children with MIS-C
Patients were categorised into three groups based on age (<5, 5–12 and 12–20 years) for subgroup comparisons. All patients were further categorised based on the requirement for mechanical ventilation. All children with MIS-C who had any residual clinical, laboratory or echocardiographic changes at the time of discharge were labelled as ‘recovered with sequelae’. The discharged patients were followed up at 6 weeks and 12 weeks to report the medium-term outcomes.
Statistical analysis
We used SPSS V.20.0 (IBM Corporation) for statistical analysis. All continuous variables were summarised using mean (SD) or median (IQR). Categorical variables were expressed in counts (%). We did a subgroup analysis by categorising the study sample based on the requirement for mechanical ventilation. We used Pearson χ2 test or Fisher’s exact test for categorical variables and independent sample t-test or Mann-Whitney test for continuous variables. We used Bonferroni’s correction for presenting p values related to multiple comparisons.
Results
Baseline characteristics
A total of 41 cases (males-23) who were diagnosed with MIS-C and treated at the two tertiary care centres from March 2020 to April 2021 were enrolled in the study.
The mean age of onset was 6.2 (4.0) years. Thirty-three (80%) cases were previously healthy whereas 8 (20%) had coexisting comorbidities. Three (8%) cases were obese, and one was overweight. Three (7%) patients who had coexisting neurological disorders—two were on antiepileptic therapy for seizure disorder, while one had congenital hydrocephalus for which surgical intervention was done. One patient had a surgically corrected congenital heart disease and one had bronchial asthma controlled on inhaled long-acting beta-agonists. (online supplemental table 1)
Supplemental material
A temporal link with COVID-19 infection was identified in all patients either in the form of serological testing or close contact with active COVID-19 case within preceding 1 month. Sixteen (39%) patients had a history of close contact with an active COVID-19 case. Two patients (5%) were having active COVID-19 infection when they developed MIS-C features, and 2 (5%) patients had previously confirmed acute COVID-19 infections and had recovered within the last 6 weeks. The first four cases (10%) did not undergo antibody assay due to regulatory restrictions on clinical use of antibody testing at that time. In the study, 28 (76%) patients were positive for COVID-19 IgG and 7 (19%) were positive for COVID-19 IgM antibody. (online supplemental figure 1)
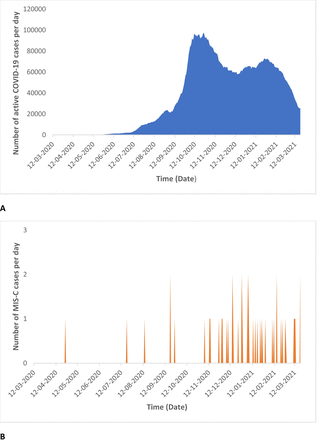
The peak of COVID-19 cases was followed by a surge in the reporting of MIS-C in November–December 2020, when the active COVID-19 cases were on a decline (figure 1A and B).
Temporal correlation of active COVID-19 cases (A) and multisystem inflammatory syndrome in children (MIS-C) cases (B) in the Southern Indian state Kerala.
Clinical characteristics
Fever was present in all patients, fatigue was in 27 (66%) and loss of appetite in 24 (59%) (table 1, figure 2A and B). The most common organ system involved was the GI system in 37 (90%) cases. Abdominal pain and diarrhoea were the most common symptoms of GI involvement seen in 32 (78%) cases each followed by nausea or vomiting in 23 (56%) cases. Pancreatitis was noted in 2 (5%) cases, and one patient (2%) had presented with appendicitis. One patient presented as intussusception. During surgical reduction, the mesenteric lymph node was biopsied, and an ill formed granuloma and neutrophilic infiltrate were detected on histopathology.
Clinical characteristics of multisystem inflammatory syndrome in children cases across the age categories*
Frequency of symptoms in multisystem inflammatory syndrome in children (MIS-C) cases (A) and cardiac assessment during acute phase of MIS-C (B).
The median duration of fever at the time of hospitalisation was 4 days (IQR 3–5) and the mean duration of GI symptoms at the time of hospitalisation was 3 (1.3) days.
The second most common manifestation was mucocutaneous involvement which was present in 36 (88%) cases. The most common mucocutaneous involvement was conjunctivitis in 29 (71%), which was bilateral nonexudative, and non-purulent. Rash was noted in 25 (61%) cases, was predominantly maculopapular rash over the trunk, extremities and periorbital region. Oropharyngeal changes including red lips/red tongue or cheilitis were present in 20 (49%) cases, and acroischaemic lesion was noted in one case.23
Thirty-two (78%) patients had both GI and mucocutaneous involvement. Muscle aches or myalgias were reported in 27 (66%) cases.
Cardiovascular system was involved in 22 (54%) cases clinically and this manifested as the presence of shock requiring inotropic agents.
Neurological symptoms were present in 21 (51%) patients. Headache was reported in 10 (24%), and meningismus in 6 (15%). Nineteen (46%) patients had either irritability, somnolence, or altered mental status, and one had ataxia.
Fifteen (37%) cases had lymphadenopathy either cervical or mesenteric. Cervical lymphadenopathy was noted on clinical examination and mesenteric lymphadenopathy was detected on radiological imaging.
Lower respiratory symptoms were present in 13 (31%) patients, shortness of breath in 12 (29%) and cough in 4 (10%). Upper respiratory symptoms were noted in 5 (12%) patients, sore throat in 3 (7%) and nasal congestion or rhinorrhoea was reported by 2 (5%). Peripheral extremity changes of oedema of hands and feet were noted in 11 (27%) patients.
Laboratory investigations and cardiac assessment
At the time of hospitalisation, anaemia was noted in 20 (49%) patients, leucopenia in 2 (5%), lymphopenia in 26 (63%), thrombocytopenia in 13 (31%) and pancytopenia in 2 (5%) patients (table 2 and table 3 cut-off values of parameters used are listed in parentheses and footnotes of tables).
Laboratory investigations of multisystem inflammatory syndrome in children cases across the age categories*†
Cardiac assessment of multisystem inflammatory syndrome in children cases across the age categories*
Among the inflammatory markers, C reactive protein (CRP) was elevated in all cases; there was a marked elevation of CRP (>100 mg/L) in 23 (56%) patients. Procalcitonin was done in 16 (39%) patients, and it was elevated in all. D-dimer was high in 40 (98%) patients, serum ferritin was high in 22 (54%) and hypoalbuminaemia was noted in 31 (76%).
Transaminitis was noted in 19 (46%) patients, acute kidney injury was identified in 4 (10%) and hyponatraemia in 9 (22%) during their hospital stay.
N-terminal pro B type natriuretic peptide (NT-proBNP) was done in 19 (46%). This was elevated in 18 (95%) patients. Troponin was done in 30 (73%) patients; it was elevated in 10 (33%). Fibrinogen was done in 11 (27%) and hypofibrinogenaemia was noted in one patient. Erythrocyte sedimentation rate (ESR) was done in 14 (34%) and it was high in 10 (71%) patients.
Cardiac assessment with an ECG and echocardiography was done in all patients. Figure 2B shows a graphical representation cardiac assessment finding. Abnormal ECG was noted in 8 (20%); bradycardia in 5 (12%), ST-T changes and complete heart block were noted in one patient each. Echocardiography was abnormal in 23 (56%) patients. CAAs were noted in 15 (37%), only hyperechoic or non-tapering coronaries in 3 (7%), dilated coronaries in 4 (10%), and small coronary aneurysms in 8 (20%) patients.
Left ventricular dysfunction was found in 10 (24%) patients—mild to moderate in 9 (22%), and severe dysfunction was noted in one (2%). There was pericardial effusion in 10 (24%), mitral valve regurgitation in 6 (15%), and global or septal hypokinesia in 4 (10%) patients.
Clinical course, treatment and immediate outcomes
A total of 36 (88%) patients required intensive care; the median duration of ICU stay was 3.5 days (IQR 3–5 days) (table 4). Twenty-one (51%) patients required inotropes and mechanical ventilation was required in 8 (20%) cases. Treatment was provided as per the standard treatment guidelines for MIS-C.6 24 25 Immunomodulatory therapy was administered to 39 (95%), steroids and IVIg both were used in 35 (85%) and only steroids were used in 3 (7%) patients. Antiplatelets were used in 37 (90%) and anticoagulation was used in 3 (7%) patients. Empirical broad-spectrum antibiotics were started for all the patients at the time of hospitalisation and were discontinued after the blood and urine cultures were noted to be sterile. (online supplemental figure 2)
Clinical course, treatment and immediate outcomes*
Two (5%) patients died during the treatment of the acute phase. The first patient was a 4-year-old girl who was positive for both COVID-19 RTPCR and antibodies with a complete heart block. She continued to deteriorate despite use of pacemaker, mechanical ventilation, supportive care and standard treatment for MIS-C. She expired on the same day of hospitalisation. The second mortality was a 17-year-old boy who had severe multiorgan dysfunction. He deteriorated rapidly despite prompt immunomodulation, hemodialysis, and mechanical ventilation. He died within 24 hours of hospitalisation.
The mean duration of hospital stay was 8.2 (4.7) days, among the patients who recovered from MIS-C. Thirteen patients (32%) recovered with some residual sequelae, primarily echocardiographic abnormalities. Remaining 26 (63%) patients recovered without any residual changes at the time of discharge (online supplemental figure 3).
Follow-up at 6 weeks after discharge
All discharged patients (n=39) remained clinically stable during 6 weeks follow-up. There was no abnormality on clinical assessment in any case. Echocardiographic assessments showed improvement trend in all patients. Eight (21%) patients had persisting coronary alterations on echocardiogram at 6 weeks—hyperechoic or non-tapering thick-walled coronaries in 5 (13%), coronary dilation in 2 (5%) and small coronary aneurysm in one patient. However, the echocardiographic coronary alterations had improved from their baseline status during the acute illness. Persisting mild left ventricular systolic dysfunction and pulmonary artery hypertension (PAH) were noted in one case each (online supplemental table 2).
Medium-term outcome at 3-months follow-up
Thirty-seven patients finished their 3-month follow-up by April 2021 (table 5). All patients were clinically stable; echocardiographic changes were improving in all of them. At 3-month follow-up, 4 (11%) patients were on Aspirin for residual coronary changes, 1 patient was on diuretics for left ventricular dysfunction, and one patient was on phosphodiesterase-5 inhibitors for PAH.
Cardiac outcomes at 3-month follow-up (n=37)*†
Comparison of MIS-C cases who required mechanical ventilation versus those who did not require mechanical ventilation
We categorised patients with MIS-C into two groups based on requirement of mechanical ventilation (table 6). We did a subgroup analysis using various clinical, laboratory and echocardiographic parameters (table 6). Only hyperferritinaemia was significantly associated with requirement of mechanical ventilation (p=0.001).
Bivariate comparison of various clinical and laboratory parameters in multisystem inflammatory syndrome in children cases who required mechanical ventilation versus those who did not require mechanical ventilation*
Discussion
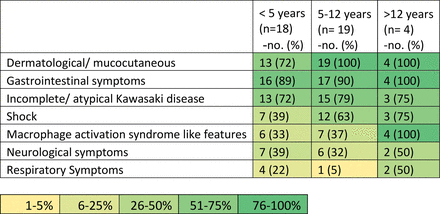
In this study, we have described 41 cases of MIS-C associated with COVID-19. The patients were treated at two tertiary care centres (Kerala, India) from March 2020 to April 2021. Figure 3 shows a heat map of syndrome clusters in MIS-C cases in our study.
Heat map of syndrome clusters based on clinical presentations. (Percentages may not total 100 because of rounding and overlapping clinical features.)
We compared our study results with those of a systematic review by Ahmed et al which involved 662 cases of MIS-C from 39 studies.9 Similarities were noted on constitutional symptoms (100% in our study vs 100% in Ahmed et al), intensive care requirement (88% vs 71%), mechanical ventilation requirement (20% vs 22%), inotrope requirement (51% vs 60%), and mean length of hospital stay (8.2 days vs 7.9 days). Laboratory parameters reflecting inflammatory, coagulative, and cardiac involvement were also similar. However, there were striking differences in our cohort which included a large number of previously healthy individuals (80% in our study vs 52). We had a smaller proportion of overweight or obese cases (10% in our study vs 24%), younger age of onset (mean age 6.2 years vs 9.3 years), high frequency of conjunctivitis (71% vs 51%), more common myalgia (66% vs 13%), more cases with irritability/somnolence (46% vs 10%), more number of cases with lymphadenopathy (37% vs 14%), and more frequent coronary dilation and aneurysms (29% vs 15%). Systolic dysfunction was less frequent in our cohort (24% vs 45%).9
We had smaller number of patients in the age group of more than 12 years in comparison with the two largest cases series from the USA (10% in our study vs 24% and 26%).26 27
There are two case series of MIS-C are reported from India. In comparison with a series of 19 patients with MIS-C from Chennai, our cohort had more frequent GI symptoms (90% in our study vs 42%), a lower proportion of individuals with active COVID-19 during MIS-C (5% vs 58%), and more common coronary involvement (37% vs 16%).28 Comparing with another case series of 23 patients with MIS-C from Mumbai, we observed a lower proportion of individuals with active COVID-19 during MIS-C (5% in our study vs 39%), higher frequency of abdominal pain (78% vs 52%), and conjunctivitis (71% vs 52%).29 Other available clinical and laboratory parameters from these two studies were similar to our study.
Because of the similarities in clinical phenotype to KD, we compared our results with existing literature on KD. We noticed that percentage of cases developing coronary artery aneurysms in our MIS-C cases were less than that of untreated KD (20% vs 25%) but more than of that KD treated with optimum IVIg (20% vs 5%). IVIg resistance or requirement of a second dose of IVIg or alternative immunotherapy was less frequent in MIS-C in comparison to KD (2% vs 10%).30 31 However, none of our current MIS-C cohort patients developed medium-sized or giant coronary aneurysm or thrombosis of coronary in contrast to KD where it is reported in 1% of treated cases.32
While it is gratifying to note that most cases remained clinically well at 3 months follow-up, the persistence of echocardiographic abnormalities in six patients emphasises the need for careful follow-up.
The only other published report on intermediate-term follow-up following MIS-C is a recently published study by Penner et al that reports outcomes at 6 months following MIS-C in a single centre cohort from the UK.33 There was similarity in context to full subsidence of inflammation (100% in our study vs 98% in Penner et al). The difference in near-complete resolution of echocardiographic sequelae (84% in our study vs 96%) could be due to our 3-month follow-up versus the 6-month follow-up in the aforementioned study. The striking difference was that in our cohort none of the patients had persistent GI symptoms, mucocutaneous changes, or minor neurological abnormalities as reported in the above-mentioned study.
MIS-C requires a high index of suspicion for diagnosis and warrants prompt treatment. There is a paucity of literature in MIS-C to define severe and non-severe cases and prediction of severity in a given case. As all of the MIS-C cases required hospitalisation, most of these required intensive care, and mechanical ventilation was required in 20% patients. We found that hyperferritinaemia was associated with the requirement of mechanical ventilation in MISC patients. Our findings are in alignment with a retrospective surveillance study from USA which enrolled 1090 patients of MIS-C.34 They reported that high ferritin in addition to high NT-ProBNP, and high D-dimer increases the odds of severe outcomes and the need for intensive care.34
As the study was conducted at two tertiary care centres it might underestimate the mild cases and could be skewed towards high morbidity and mortality due to referral bias.
Conclusions
MIS-C is a new disease in context to COVID-19 pandemic and we are still continuing to learn about this clinical syndrome.
While the clinical profile of our cohort has largely been similar to worldwide reports, we observed a few differences in our cohort like younger age at onset, more mucocutaneous changes and a smaller number of patients with coexistent comorbidities. Risk factors for the development of severe MIS-C remain unknown; however, we found that requirement of mechanical ventilation was associated with hyperferritinaemia.
We observed echocardiographic sequalae in one-third of patients at the time of discharge, which reduced to one in six at 3-month follow-up. Overall immediate and medium-term outcomes remain largely excellent. Ongoing follow-up for several years to study the disease’s natural history is certainly warranted.
Data availability statement
Data are available upon reasonable request. Please contact sumabalan@gmail.com for any request related to data sharing.
Ethics statements
Patient consent for publication
Ethics approval
This study was approved by the institutional ethics committee: Institutional Review Board of Amrita Institute of Medical Sciences (IRB-AIMS-2020-335) which involved public representatives as well. A written informed consent was obtained from the parents of study participants
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Twitter @DrArunTiwari1, @doc_manu
Contributors AT took the lead in data curation, formal analysis, software and writing original draft. SB and AR took the lead in conceptualisation. SB took the lead in project administration, investigations, resources and supervision. MR, SB and MK took lead in writing review and editing the manuscript. AR, MK, MR, SK, SS, AKV, PC, AV, STJ, SCK, RAK, and AS provided critical feedback in conceptualisation, methodology, drafting and revisions which helped to shape the manuscript.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.