Article Text
Abstract
Background In adults, there is increasing evidence for an association between antibiotic use and gastrointestinal (GI) disorders but in children, the evidence is scarce.
Objective Assess the association between exposure to antibiotics in the first 2 years of life in term born children and the presence of chronic GI disorders later in childhood.
Design For this systematic review the MEDLINE, Embase, WHO trial register and Web of Science were systematically searched from inception to 8 June 2020. Title and abstract screening (n=12 219), full-text screening (n=132) as well as the quality assessment with the Newcastle-Ottawa Scale were independently performed by two researchers.
Main outcome measures The association between antibiotics and inflammatory bowel disease (IBD) (n=6), eosinophilic oesophagitis (EoE) (n=5), coeliac disease (CeD) (n=6), infantile colics (n=3), functional constipation (n=2), recurrent abdominal pain, regurgitation, functional diarrhoea and infant dyschezia were examined.
Results Twenty-two studies were included, 11 cohort and 11 case–control studies. A best evidence synthesis showed strong evidence for an association between antibiotic exposure in the first 2 years of life and the presence of IBD, and CeD during childhood. Moderate evidence was found for an association with EoE and no association with functional constipation in the first year of life. There was insufficient evidence for the other studied disorders.
Conclusions The use of antibiotics in early life may increase the risk of GI disorders later in life. Further studies are necessary to unravel the underlying mechanisms and determine potential preventive measures. Meanwhile judicious use of antibiotics in early childhood is highly warranted.
PROSPERO registration number PROSPERO CRD42019132631.
- gastroenterology
- epidemiology
- neonatology
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
What is known about the subject?
Evidence about the association between antibiotic use and gastrointestinal (GI) disorders is increasing for adults, but in children the evidence remains scarce.
The incidence of GI disorders in childhood is increasing.
What this study adds?
Antibiotics in early life may increase the risk of GI disorders later in life especially inflammatory bowel disease and coeliac disease.
Although functional GI disorders are the most frequent in childhood, very few studies examined their association with antibiotics in early life.
Introduction
The incidence of paediatric gastrointestinal disorders (GI disorders), such as paediatric inflammatory bowel disease (IBD) and coeliac disease (CeD), is rising.1 2 The increase in paediatric GI disorders is most likely related to environmental factors and recently the focus has been on the role of the intestinal microbiome. A microbiome that has been disturbed by factors like stress, dietary change, environmental factors or drugs, can result in alterations in the immune system.3 Several studies have shown that a disturbed microbiome can be a cause or trigger of GI disorders, probably mediated by these immunological changes.4–7
One of the drugs with the most profound effect on the microbiome are antibiotics.8 The impact of antibiotics on the microbiome depends on various factors such as type of antibiotic, dosage and duration of exposure.8 Furthermore, age at exposure is probably also important. The gut of a newborn infant is almost sterile with a low diversity and matures according to several developmental stages with increasing diversity over time.9 The microbiome stabilises around the age of 2–3 years.9 Since this developing gut microbiota plays an important role in the training of both innate and adaptive immune system, it is likely that antibiotics will have their biggest impact when administered in the first 2 years of life.
For the association between antibiotic use and GI disorders, that has been shown in adults,10 there is only limited evidence in children.11 Therefore, the aim of this systematic review was to assess the association between exposure to antibiotics in the first 2 years of life and the presence of chronic GI disorders during childhood.
Method
Study selection
This systematic review was conducted according to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses and registered in PROSPERO CRD42019132631.12 13 MEDLINE, Embase, WHO trial register and Web of Science were systematically searched from inception to 8 June 2020 to identify all studies examining the association between antibiotic exposure in the first 2 years of life and the presence of common chronic (longer than 2 weeks, in order to exclude viral diarrhoea) GI disorders during the first 18 years of life. We searched for associations with IBD, eosinophilic oesophagitis (EoE), CeD, irritable bowel syndrome (IBS), (functional) abdominal pain (AP), constipation, dyspepsia, aerophagia, infantile colic, gastro-oesophageal reflux (GERD), regurgitation, dyschezia and chronic diarrhoea.
A multi stranded search approach comprised various concept combinations of children aged 0–4 years, prognosis, GI disorders and antibiotics. In order to reduce recall noise and enhance search results precision we used VOS-viewer to identify terms for NOTing out irrelevant records from databases searched.14 15 See online supplemental file 1 for the full search strategies.
Supplemental material
Patient and public involvement statement
As this is a systematic review of the literature, there were no patients involved in the design of the research question nor the study itself. Furthermore, for the same reason no approval for the study was required from an ethical committee.
In- and exclusion criteria
Studies were included if: (1) antibiotics were administered between full-term birth and 2 years of age; (2) study outcome was diagnosis with a chronic GI disorder during the first 18 years of life; (3) antibiotic use was before the diagnosis of the GI disorder; (4) a control group was included; (5) in case multiple studies were found examining similar outcomes in one cohort, only the study with the largest cohort was included. No restrictions were placed on the time period of publication. Searches were limited to studies conducted in humans and excluded if the full text was not available in English, Dutch, German or French.
All records found in the search were exported into Rayyan after deduplication.16 Two researchers (KK and EVD) independently performed title and abstract screening as well as full-text screening. After consensus about the study selection, data were entered into a data extraction form, which included: author, year of publication, country, study design, cases, controls/cohort, population age, sample size exposed to antibiotics, age at exposure, details about classification by type of antibiotics, type of GI disorder, method of diagnosis, confounders for which corrected and the association between exposure and outcome.
Methodological quality
To assess the risk of bias, two researchers (KK and EVD) independently assessed the methodological quality. Discrepancies were resolved by discussion until consensus was reached. The Newcastle-Ottawa Scale (NOS) was used, which has been developed to assess the quality of observational studies.17 The NOS includes different instruments for assessing case–control and cohort studies. Both scales contain a maximum of nine points and assess studies in three core areas: (1) selection of study participants; (2) comparability of groups; (3) detection of exposure/outcome. One point for comparability of groups was given when the study controlled for the main important confounder and a second point if controlled for a second important confounder, see online supplemental file 2. Studies were rated high quality with a score of eight or higher, moderate quality with a score between five and seven and weak quality with a score of four or less.18
Supplemental material
Data analyses
To synthesise the methodological quality of the studies, a commonly used best evidence synthesis was applied per disorder in which the methodological quality was considered according to the following definitions: (1) strong evidence, provided by generally consistent findings in at least two high-quality studies; (2) moderate evidence, provided by generally consistent results in one high-quality study and at least one moderate-quality or low-quality study, or generally consistent results in multiple moderate-quality or low-quality studies; (3) insufficient evidence, when less than two studies were available or inconsistent findings in multiple studies.19–21 Results were considered consistent when at least 75% of the studies showed results in the same direction.
Results
Search results
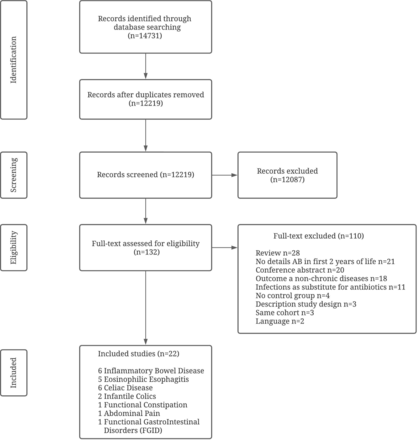
Of the 14 731 retrieved records, 12 219 remained after removing duplicates. These records were screened; 132 were assessed as eligible and read in full-text of which 110 were excluded and 22 studies included in this review. Details of the selection procedure are shown in figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram of the study selection.
Study characteristics
The included studies were published between 2010 and 2020 (tables 1A–D): 11 cohort studies22–32 and 11 case–control studies.33–43 The studies were performed in Sweden (n=4),27 30 35 36 the USA (n=5),33 34 37 41 42 Italy (n=4),22 29 32 43 Denmark (n=2),23 31 Canada (n=2)38 39 and one in the UK,25 the Netherlands26 and Finland.40 There were two international studies, one in Denmark and Norway,28 and another in Finland, Germany, Sweden and the USA.24
Study characteristics and association with antibiotics: inflammatory bowel disease
Study characteristics and association with antibiotics: eosinophilic oesophagitis (EoE)
Study characteristics and association with antibiotics: coeliac disease (CeD)
Study characteristics and association with antibiotics: FGIDs: infantile colics, functional constipation (FC), recurrent abdominal pain (AP) and regurgitation, functional diarrhoea and infant dyschezia
The associations between antibiotics and the following GI disorders were examined: IBD (n=6),25 27 31 38 40 43 EoE (n=5),33 34 37 39 41 CeD (n=6),22 24 28 35 36 42 infantile colics (n=3),23 26 32 functional constipation (n=2),29 32 recurrent AP (n=1).30 One study examined several functional GI disorders (FGIDs): infantile colics, functional constipation, functional diarrhoea, infant dyschezia and regurgitation.32
Exposure to antibiotics was studied in the first 2 years of life (n=4),24 30 35 42 the first 18 months of life (n=1),23 the first year of life (n=13),22 25 27–29 31 33 34 37–40 43 the first 6 months of life (n=2)36 41 and the first week of life (n=2)26 32 (tables 1A–D). Since only a few studies provided details about type of antibiotics and/or number of antibiotic treatments in the first 2 years of life, the associations include mostly the overall antibiotic exposure.
Quality assessment
Ten studies were of high quality,22 26–29 31 35 38 40 43 ten studies moderate23–25 30 32 34 36 37 41 42 and two weak33 39 (table 2). Frequently observed weaknesses were a high dropout rate in the cohort studies, assessment of antibiotic exposure through parental reports, and no correction for important confounders.
Quality assessment
Inflammatory bowel disease
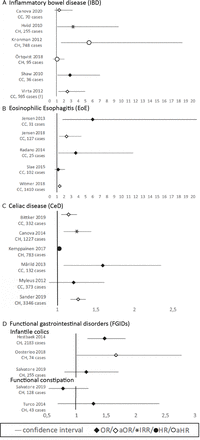
Exposure to early life antibiotics was associated with the development of IBD in five out of six studies25 31 38 40 43 (NOS=7,8,8,8,8), whereas no association was found in one study examining very early onset (VEO) IBD (before 6 years of age)27 (NOS=8). Three studies found a dose–response relation25 38 43 and an increased risk after fluoroquinolone,25 metronidazole25 and phenoxymethylpenicillin40 exposure. In two studies IBD was stratified by type and only the OR for Crohn’s disease, but not for ulcerative colitis, was significant.38 40 Forest plots of the main results are shown in figure 2A.
Forest plots per gastrointestinal disorder. (A) IBD; (B) EoE; (C) CeD; (D) FGID (infantile colics and functional constipation). CC, case control study, CH, cohort study, (!) Virta 2012 only shows the results of the phenoxymethylpenicillin analyses, overall use of antibiotics was not significant.
Eosinophilic oesophagitis
In four of the five studies early life antibiotics was associated with EoE33 34 37 41 (NOS=4,6,7,7), whereas in one study the rates of parental reported antibiotic use were similar for cases and controls39 (NOS=3) (figure 2B).
Coeliac disease
In four studies, of which three had a high quality, a significant association between early life antibiotics and the presence of CeD was found22 28 35 42 (NOS=8,9,8,5), whereas in two moderate quality studies no association was found24 36 (NOS=6,7) (figure 2C). Three studies showed a dose–response relationship between exposure to antibiotics and the risk of CeD.22 28 42 Furthermore, use of cephalosporin22 and multiple courses of macrolides24 showed a positive association with the development of CeD.
Infantile colics
Two studies found a significant association between early life antibiotics and infantile colics23 26 (NOS=6,8), while one study found no association32 (NOS=7) (figure 2D).
Functional constipation
In both studies, no association was found between early life antibiotic use and functional constipation in the first year of life29 32 (NOS=8,7).
Recurrent AP
The only study examining the association between antibiotic use in the first 2 years of life and the risk of recurrent AP at 12 years of age30 (NOS=5) found that only girls, but not boys, who received antibiotics in both the first and second year of life, had an increased risk of AP at 12 years.
Regurgitation, dyschezia and functional diarrhoea
In one study no association was found between antibiotics in the first week of life and regurgitation, dyschezia and functional diarrhoea32 (NOS=7).
Syntheses of individual results
Using the definitions for the best evidence synthesis, described in the method section, it can be concluded that there is strong evidence for an association of antibiotics in early life with IBD and CeD. There is moderate evidence for an association with EoE and no association with infantile constipation. The current evidence for an association between antibiotics in early life and the other studied GI disorders is considered insufficient.
Discussion
This systematic review with best evidence syntheses on the association between antibiotic exposure in the first 2 years of life and chronic GI disorders during childhood showed strong evidence for this association with IBD and CeD, and moderate evidence for this association with EoE. For the other studied GI disorders, insufficient evidence was found.
The question remains to what extent the association with IBD, EoE and CeD can be attributed to antibiotic exposure itself or to other factors such as infections and parental health seeking behaviour. Infections in early life have been proposed to contribute to the development of chronic GI disorders44 45 and it is difficult to differentiate between the role of infections and antibiotics which are prescribed for (suspected) infections. Furthermore, several GI disorders like CeD can remain undiagnosed for a long time. Higher parental health seeking behaviour can both lead to higher use of antibiotics and a higher chance of diagnosing the chronic GI disorder. Therefore, it remains unknown whether antibiotics are the true causative agent in the observed associations or whether they are intermediates in different mechanistic pathways through microbiome perturbations or changes in immune development after (suspected) infections.
Most studies found a clear association between antibiotics in early life and IBD. The study that focused on VEO-IBD, found no association between antibiotics and VEO-IBD. VEO-IBD is considered a different entity from later-onset IBD,44 since genetics play a far more important aetiological role than microbial dysbiosis.45 This may explain the lack of an association with early life antibiotics.
The primary goal of antibiotic administration is to prevent detrimental effects of serious and sometimes even life-threatening infections. However, especially in early life, antibiotics are overused, since they are often prescribed for viral upper respiratory tract infections.46 47 Given its association with the occurrence of IBD, CeD and EoE, it is highly important to prevent antibiotic overuse by strict adherence to guidelines. If antibiotics are necessary, treatment would be adjusted to minimise dysbiosis. Another possible solution is to shorten the time of antibiotic administration. Oosterloo et al found more health issues in the first year of life after 7 days compared with 2 days of antibiotics in the first week of life.26 Furthermore, whenever possible, narrow-spectrum antibiotics rather than broad-spectrum should be used, because these specifically reduce the capacity of pathogens to cause disease while leaving commensals unharmed.48 If adjustment of antibiotic treatment is not possible, interventions that restore or prevent dysbiosis should be considered, such as administration of prebiotics or probiotics or faecal transplants.49–52
Some limitations of this review need to be considered. As no randomised controlled trials were available, only associations but not causality can be examined. Additionally, the studied results were not evaluated for their precision and associations with wide CIs can indicate uncertainty about the magnitude of the association. Hence, the results must be interpreted with caution. Furthermore, both age at exposure as well as age at diagnosis varied substantially between the studies. In addition, study outcomes were also very heterogeneous, excluding a meta-analysis. Therefore, a best evidence synthesis was applied, taking the quality of the studies into account. Furthermore, the recording of antibiotic exposure was in half of the studies parental reported, which may have led to recall bias. The antibiotics were mostly analysed as overall use, without distinguishing between types of antibiotics and therefore, it was not possible to determine associations between certain type of antibiotics and GI disorders. Finally, for several functional GI disorders, like IBS or GERD, only few or even no studies were found which prohibits any conclusions on these GI disorders.
One of the strengths of this review is that the search string was built and performed by an information scientist. Besides the published articles, also conference abstracts were checked for relevant studies. Furthermore, this review studies the association between antibiotics in early life and all chronic GI disorders in childhood, which provides insights in the available evidence but also shows the gap of knowledge for these associations.
For future research, it is recommended to study the association between early life antibiotics and the presence of those GI disorders that currently lack sufficient studies. Furthermore, it is necessary to gain insights in the specific effect of different types of antibiotics on the microbiome in order to optimise therapies that can prevent or counteract the detrimental effects of antibiotics in early life.
Conclusion
This systematic review shows strong evidence for an association between antibiotic exposure in the first 2 years of life and the presence of IBD and CeD later in childhood. For the other included GI disorders, only moderate or insufficient evidence was found. In order to decrease the incidence of IBD and CeD, antibiotic administration in early life should be critically considered. Moreover, interventions need to be developed to restore the microbiome after unavoidable antibiotic exposure in order to prevent detrimental health consequences later in life.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
KK and EVD are joint first authors.
KK and EVD contributed equally.
Contributors KK contributed to the design, the analyses and interpretation of the study, drafting of the initial manuscript, and reviewed and revised the manuscript. EVD contributed to the analysis and interpretation of the study and critically revised the manuscript. AMV and RMvE contributed to the conception of the study, interpretation of the data and critically revised the manuscript. JGD conceptualised and performed the systematic search and critically revised the manuscript. JK contributed to the conception and design of the study and critically revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Funding This study was funded by Nutricia Netherlands B.V. as part of a public private partnership and by the Christine Bader foundation (CBSIKZ).
Competing interests JK is a fulltime employee of Danone Nutricia Research (DNR), the PhD trajectory of EVD and KK are partly sponsored by DNR.
Patient consent for publication Not required.
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement Data sharing not applicable as no datasets generated and/or analysed for this study. N/A.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.